
Neuraxial anesthesia and analgesia for labor and cesarean delivery.
In recent years, regional anesthesia techniques for surgery, obstetrics, and postoperative pain management have been used with increasing frequency.(1–3) The combined spinal-epidural (CSE) technique, a comparatively new anesthetic choice, includes an initial subarachnoid injection followed by epidural catheter placement and subsequent administration of epidural medications. This allows for rapid relief of pain or induction of regional anesthesia by the rapid onset of the spinal drugs and subsequent administration of medications for prolonged anesthesia. In addition, postoperative analgesia via the epidural catheter can be delivered for extended periods.
Clinical studies have demonstrated that the CSE technique provides excellent surgical conditions as quickly as the single-shot subarachnoid block and with advantages in comparison to the conventional epidural block. The advantage lies in the fact that CSE anesthesia offers benefits of both spinal and epidural anesthesia.
Although the CSE technique has become increasingly popular over the past two decades, it is a more complex technique that requires a comprehensive understanding of epidural and spinal physiology and pharmacology.
This course discusses the technical aspects, advantages, potential complications, and limitations of the CSE technique for surgery, postoperative pain management, and labor analgesia.
/Chapter 19: CSE/CH19_CSE - Clinical Application and Advantages.jpg)
The results of a survey conducted by Blanshard and Cook demonstrated wide variation in CSE anesthesia use and practice among experienced anesthesiologists,(7) reflecting concern over the frequency of CSE-related complications,(8,9) controversy over the technique(10,11), and the potential for higher failure rates with the CSE technique compared with individual spinal or other anesthetic techniques.
The CSE technique has been described in the medical literature for use in general surgery, orthopedics, trauma surgery of a lower limb, and urological and gynecological surgery. Clinical studies have demonstrated that the CSE technique provides excellent surgical conditions as quickly as with single-shot subarachnoid block—conditions that are better than with epidural block alone.(4,15) With the CSE technique, surgical anesthesia is established rapidly, saving 15–20 minutes compared with epidural anesthesia. Furthermore, epidural catheterization provides the possibility of supplementing the subarachnoid anesthesia, which may be insufficient when used alone.
This was recently illustrated by Mane et al,(14) who presented a case series of laparoscopic appendectomy successfully performed under CSE anesthesia. CSE anesthesia in their series was performed using separate needles at two different interspaces. Spinal anesthesia was performed at the L2–L3 interspace using 2 mL 0.5% (10 mg) hyperbaric bupivacaine mixed with 25 μg fentanyl. The epidural catheter was inserted at the T10–T11 interspace to supplement spinal anesthesia and for postoperative pain relief. In an obstetrics-related article, it was also observed that various needles can be used in different combinations when performing the CSE technique and may have different advantages and disadvantages for different patients and situations.(15)
The CSE technique is widely used in obstetric practice to provide optimal analgesia for parturients. It offers effective, rapidonset analgesia with minimal risk of toxicity or motor block.(16) In addition, this technique provides the ability to prolong the duration of analgesia, as often is required in labor, through the use of an epidural catheter. Furthermore, should an operative delivery become necessary, that same epidural catheter can be used to provide operative anesthesia. The onset of spinal analgesia is almost immediate, and the duration is between 2 and 3 hours, depending on which agent or agents are chosen.
The duration of spinal analgesia, however, has been found to be decreased when administered to a woman in advanced labor versus one in early labor.(17) Laboring patients may have greater satisfaction with CSE anesthesia than with standard epidurals, perhaps because of a greater feeling of self-control.(18) The original descriptions of spinal labor analgesia utilized sufentanil or fentanyl,(19) but the addition of isobaric bupivacaine to the opioid produces a greater density of sensory block while still minimizing motor block.(20) Originally, 25 μg of fentanyl or 10 μg of sufentanil were advocated, but subsequent studies suggested the use of smaller doses of opioid combined with a local anesthetic.(21) For example, many clinicians are now routinely using 10–15 μg of intrathecal fentanyl. Several studies have suggested that ropivacaine and levobupivacaine can be substituted for intrathecal bupivacaine, especially when added to an opioid, to provide labor analgesia.(22–24) The CSE technique has also made ambulation possible for many women receiving neuraxial analgesia, although ambulation may also be possible with other techniques. Wilson et al(25) showed that significantly more women maintained superior leg power for a longer period with CSE anesthesia than with low-dose infusion of standard epidural. In addition to the advantage of rapid onset of pain relief, the CSE technique may reduce the incidence of several potential problems associated with the conventional epidural technique, including incomplete (patchy) block, motor block, and poor sacral spread.
Another potential advantage of the CSE technique is that it may be associated with a significant reduction in the duration of the first stage of labor in primiparous parturients.(26,27) However, according to a more recent study by Pascual-Ramirez et al,(28) when compared with conventional epidural analgesia, the CSE technique did not shorten total labor duration but did reduce local anesthetic requirement and motor weakness. Reduction of motor block is advantageous for parturients, even those who will not ambulate.
The CSE technique, first reported as an option for cesarean delivery in 1984,(29) has recently increased dramatically in popularity. The advantage of this technique is that it provides rapid onset of dense surgical anesthesia while allowing the ability to prolong the block with an epidural catheter. In addition, because the block can be supplemented at any time, the CSE technique allows the initial use of smaller doses of spinal local anesthetics, which may in turn reduce the incidence of high spinal block or prolonged hypotension.(30) It may also reduce the duration of the post-anesthesia care unit (PACU) stay. Potential problems of the CSE technique for cesarean delivery include an inability to test the catheter, the possibility of a failed epidural catheter after spinal injection, and the risk of enhanced spread of previously injected spinal drug after use of the epidural catheter.(31)
Neuraxial analgesia has been used to reduce the maternal pain during external cephalic version (ECV) for breech presentation. A potential benefit of the CSE technique is the ability to provide fast and effective pain relief for ECV and convert to neuraxial anesthesia for emergency delivery, if required. Kawase et al (32) reported a successful case of ECV under CSE technique followed by vaginal delivery. Sullivan and collegues (33) studied the effect of the CSE technique on the success of ECV when compared with systemic opioid analgesia and found no difference; however, the pain scores were lower and satisfaction was higher with CSE analgesia.
When CSE block was compared with either epidural or subarachnoid block for hip or knee arthroplasty, CSE anesthesia was found to be superior to epidural anesthesia. With the CSE technique, surgical anesthesia was rapidly established, saving 15–20 minutes compared with epidural anesthesia. Furthermore, the epidural catheter provided the possibility of supplementing insufficient subarachnoid anesthesia. Patients who received the CSE technique had more intense motor block than those who received epidural anesthesia alone. (4)
It has been reported that the CSE technique decreases the failure rate and incidence of several other adverse events associated with neuraxial analgesia. (34) In a retrospective analysis of almost 20,000 deliveries (75% neuraxial labor analgesia rate), the overall failure rate with this technique was 12%. The patients had adequate analgesia from initial placement, but 6.8% of patients had subsequent inadequate analgesia during labor and required epidural catheter replacement. Ultimately, 98.8% of all patients in Pan’s report received adequate analgesia, even though 1.5% of patients had one or more epidural catheter replacements. (34) However, when compared with epidural analgesia alone for labor, the incidents of overall failure, accidental intravascular epidural catheters, accidental dural punctures, inadequate epidural analgesia, and catheter replacements were repeatedly shown to be significantly lower in patients receiving CSE analgesia.(16,34,35) In addition, Eappen et al reported that CSE had a higher success rate compared to the conventional epidural technique. (35) This difference may be due to the ability to confirm questionable epidural location by successful spinal placement and observation of cerebrospinal fluid (CSF).
CSE enables low-dose spinal anesthesia for cesarean delivery.(36–40) When using single-shot spinal (SSS) anesthesia for ambulatory surgery, many anesthesiologists tend to administer more medication than is needed because there is only one chance to ensure an effective spinal block. The presence of an epidural catheter as a “safety net” allows the anesthesiologist to use the lowest effective dose of local anesthetic. Urmey et al used the CSE technique to investigate the appropriate dose of intrathecal isobaric lidocaine 2% for day-case arthroscopy. (41) The CSE technique provided excellent anesthesia for all 90 patients in his study. Patients receiving the smallest dose (40 mg) had a significantly shorter duration of anesthesia, which allowed quicker discharge than for the patients receiving 60 or 80 mg of intrathecal lidocaine.
Norris et al suggested the use of a CSE technique with intrathecal sufentanil alone for outpatient shock-wave lithotripsy, reserving the use of epidural catheter for patients who did not achieve adequate analgesia. (42)
Patel et al (43) studied the impact of spinal medication administered as part of a CSE technique on the subsequent epidural bupivacaine requirement. In a prospective, randomized, double-blind study, the MLAC (minimum local analgesic concentration) of epidural bupivacaine for labor analgesia was assessed following initial intrathecal (CSE) or epidural medication (standard epidural). They reported that the MLAC of epidural bupivacaine was not reduced by the use of intrathecal medication, but actually increased by a factor of 1.45. (MLAC in the standard epidural group was 0.032% wt/vol and for the CSE group was 0.047% wt/vol.) This suggests that CSE analgesia may not offer a quantitative analgesia advantage over standard epidural analgesia beyond the initial dose.
During CSE anesthesia, it has been shown that supplementation of the epidural space with epidural saline (“epidural volume extension,” EVE) may influence the anesthetic level and quality of spinal anesthesia. The proposed mechanism for this augmentation is a compressive effect on the subarachnoid space that promotes the cephalad spread of local anesthetic.
Takiguschi et al,(44) in a study using myelography on human volunteers, demonstrated that the contrast medium in the subarachnoid space was displaced cranially after lumbar epidural saline injection and the diameter of the subarachnoid space was narrowed due to the volume effect. This is a time-dependent phenomenon with maximum benefit if performed early.
Similarly, Blumgart et al showed that EVE with 10 mL of normal saline resulted in an increase in sensory block height of four segments following the administration of 8–9 mg of subarachnoid hyperbaric bupivacaine in women undergoing cesarean delivery.(45) However, a more recent study by Loubert et al failed to show a difference in sensory block height after EVE with 5 mL of normal saline.(46) It is possible that the 5-mL volume was insufficient in this patient population, although the volume of normal saline that has been previously shown to be effective for EVE is approximately 5–10 mL.(47)
The results may also be due to a positional effect; in both studies, the CSE technique was performed in the sitting position. However, Blumgart injected 10 mL of saline epidurally through the catheter within 5 minutes of hyperbaric spinal medication only after the patient was turned supine with a 15° left lateral tilt. In Loubert’s study, 5 mL of normal saline were injected through the Tuohy needle immediately after the spinal hyperbaric medication while the patients were still in the sitting position. Finally, the epidural catheter was threaded and the patients were helped into the supine 15° left lateral tilt.
Is the baricity of the local anesthetic a factor? A study by Tyagi et al demonstrated (in nonobstetric patients) that EVE was more effective with plain bupivacaine compared to hyperbaric bupivacaine, requiring a smaller dose while producing a higher sensory block with an earlier onset.(48) They attributed this difference to the restricted spread of hyperbaric local anesthetics in the subarachnoid space compared to the plain solution. Another study by Tyagi et al found that the intrathecal block level was similar in duration and extent with hyperbaric bupivacaine whether given as a SSS or a CSE administration with or without EVE on parturients undergoing elective cesarean delivery.(49)
Many factors seem to affect EVE. These include timing, volume of saline, features of the local anesthetic (hyperbaric vs. hypobaric), position during or after spinal anesthesia, and obstetric versus nonobstetric patients. Although it has been proposed that EVE may allow a reduced subarachnoid dose of local anesthetic for surgery and consequently reduce the incidence of hemodynamic effects associated with spinal block, there is a lack of uniformity between protocols and study results. Therefore, the influence of epidural saline injection on the quality of spinal anesthesia remains unclear.
In a study by Fan et al, four different intrathecal doses of hyperbaric bupivacaine (2.5, 5, 7.5, and 10 mg) were compared in patients undergoing cesarean delivery under sequential CSE block, a technique that involves administration of a relatively small subarachnoid block that may be supplemented as needed by epidural local anesthetics. The authors demonstrated that 5 mg intrathecal bupivacaine combined with an appropriate dose of epidural lidocaine provided adequate surgical analgesia while maintaining optimal hemodynamic stability. Higher doses of intrathecal bupivacaine were associated with typical adverse effects of high subarachnoid block, such as nausea, vomiting, and dyspnea.(50)
Macfarlane et al demonstrated that CSE anesthesia appears to offer no hemodynamic benefits compared with SSS anesthesia during cesarean delivery when the same dose of local anesthetic is administered. Hemodynamic stability was studied directly by measuring noninvasive blood pressure and indirectly by the ephedrine requirement, systemic vascular resistance index, and cardiac index using thoracic impedance cardiography.(51)
The sequential CSE technique may be particularly advantageous in high-risk patients, such as those with cardiac disease, when slower onset of sympathetic block is desirable.(52,53) Most spinal anesthetics are administered as a single-injection procedure, and rapid onset of sympathetic block may result in abrupt, severe hypotension. Traditionally, high-risk patients are managed with the slow onset of controlled epidural anesthesia, which requires much higher total dosages of local anesthetic than is the case with sequential CSE. With careful positioning of the patient prior to induction of the subarachnoid block, and by allowing titration with small incremental epidural doses to the precise level of anesthesia desired, the sequential CSE technique may enhance the safety of the neuraxial block.
Agarwal et al reported successful management of hysterectomy in a patient with ventricular septal defect and pulmonary atresia (VSD-PA) using CSE with the EVE technique.(54) Along similar high-risk lines, Month et al presented two parturients with idiopathic intracranial hypertension who achieved both labor analgesia and symptomatic relief using the CSE technique with small-volume CSF withdrawal.(55)
In summary, CSE can reduce or eliminate many of the disadvantages of subarachnoid or epidural anesthesia alone while preserving their respective advantages. The CSE block offers the speed of onset, efficacy, and minimal toxicity of a subarachnoid block combined with the potential of improving an inadequate block or prolonging the duration of anesthesia with epidural supplements; with the epidural, one may extend the analgesia well into the postoperative period. Although the sequential CSE technique will take somewhat longer than the standard CSE technique, the use of minimal doses of local anesthetics has been shown to reduce the frequency and severity of hypotension when compared with epidural or spinal techniques.(56)
Despite numerous studies advocating CSE, a 2007 Cochrane review of 19 randomized trials involving 2658 laboring women concluded that CSE offers little benefit when compared to conventional epidural analgesia, and there was no difference in the overall satisfaction of the women between the two techniques.(57) However, the authors did acknowledge that CSE produced slightly faster onset of effective pain relief and less need for rescue analgesia and was associated with less urinary retention. Later, Van de Velde(58) criticized this Cochrane review, stating that a number of well-performed studies were excluded from the analysis. He wrote, “With conventional epidural analgesia, a wider interpatient variability exists with respect to onset time of analgesia. With CSE, onset time is short in all patients irrespective of the other factors.”
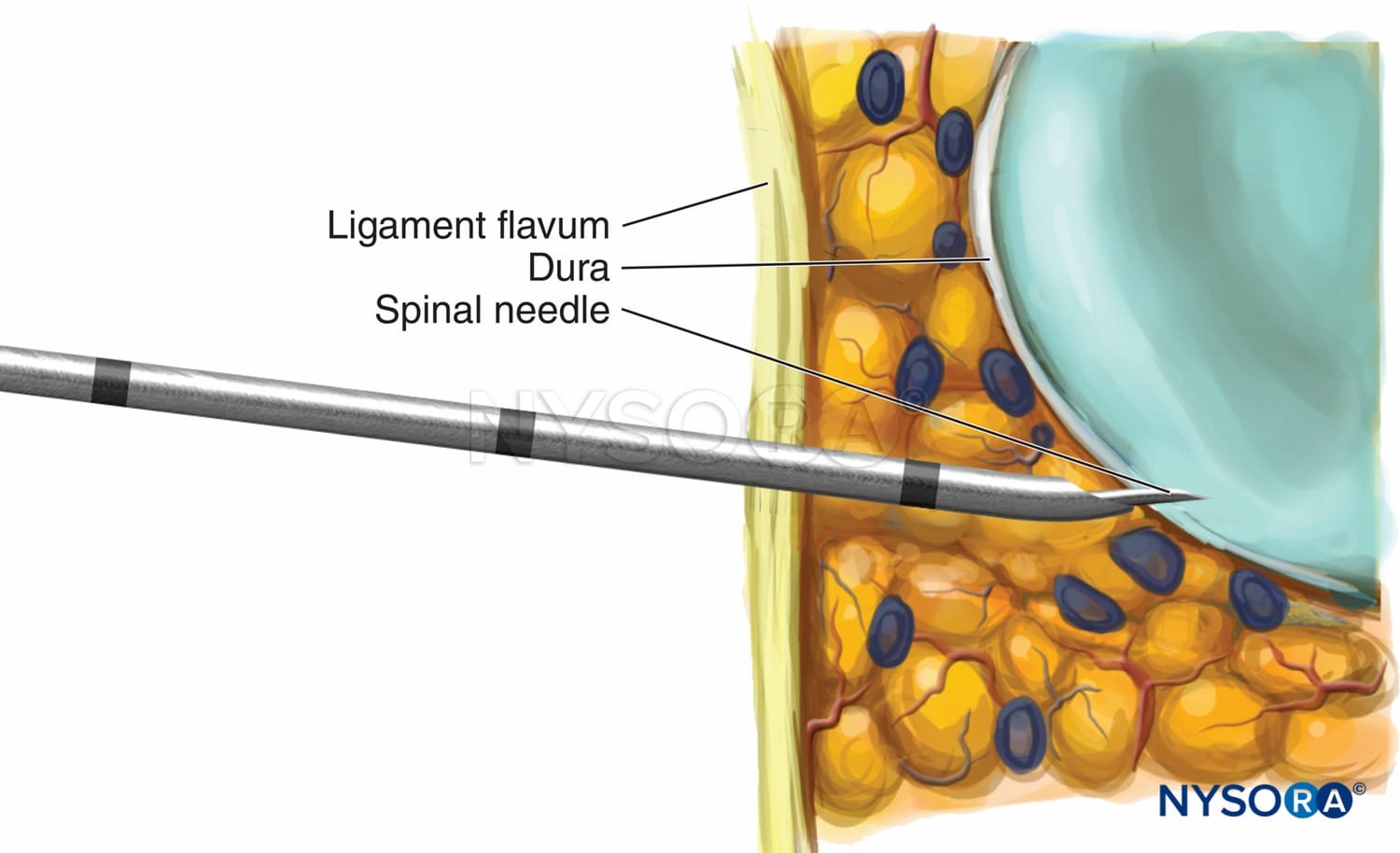
When performing an epidural block, skin-to-epidural space distance (SED) and the posterior epidural space distance (PED) are measures that can help reduce the inadvertent penetration of the dura and injury to neural structures.(59,60) The knowledge of these distances is also important in the success rate of epidural blocks. The PED, a measure of the epidural space depth, is particularly important with the CSE needle-through-needle (NTN) technique. Underestimation of this distance (short protrusion of the spinal needle through the epidural needle) will result in a higher incidence of spinal block failure. Any nonmidline approach also would increase the risk of not reaching the subarachnoid space because the dural sac has a triangular shape with the top pointing dorsally. Overestimation of PED will cause over protrusion of the spinal needle, which may increase the risk of neural damage.(61) These distances have been measured using various methods,(62) including magnetic resonance imaging (MRI), computed tomography (CT), ultrasound, and measurement of CSE tip-to-tip distance or the amount of protrusion of the spinal needle beyond the Tuohy needle.
The distance from the SED is most commonly 4 cm (50%) and is 4–6 cm in 80% of the population according to detailed records of 3200 cases.(62) The width of the PED varies with vertebral level, being the widest in the midlumbar region (5–6 mm) and decreasing toward the cervical vertebral column. In the midthoracic region, it is 3–5 mm in the midline and narrows laterally. In the lower cervical region, it is only 1.5–2 mm in the midline.(63) These spaces also correlate with the weight/ height ratio and body mass index (BMI).(64) Based on these measures, the present design of spinal needle protrusion varies between 10 and 15 mm beyond the epidural needle.
The thickness of the ligamentum flavum, distance to dura, and skin-to-dura distance vary with the area of vertebral canal (see Table 1).
| Table 1. Characteristics of ligamentum flavum at different vertebral levels. | ||
|---|---|---|
| Site | Skin to ligament (cm) | Thickness of ligament (mm) |
| Cervical | - | 1.5 - 3.0 |
| Thoracic | - | 3.0 - 5.0 |
| Lumbar | 3.0 - 8.0 | 5.0 - 6.0 |
| Caudal | Variable | 2.0 - 6.0 |
The two ligamenta flava are variably joined (fused) in the midline, and this fusion or lack of fusion of the ligamenta flavum occurs at different vertebral levels in individual patients. Lirk et al investigated the incidence of lumbar ligamentum flavum midline gaps in embalmed cadavers.(65) Vertebral column specimens were obtained from 45 human cadavers. The gaps in the lumbar ligamentum flavum are most frequent between L1 and L2 (22.2%) but are rare below this level (L2–L3 = 11.4%, L3–L4 = 11.1%, L4–L5 = 9.3%, L5–S1 = 0). Therefore, when using a midline approach, one cannot rely on the ligamentum flavum to impede entering the epidural space in all patients.
/Chapter 19: CSE/CH19_CSE - Techniques.jpg)
A number of reviews have discussed the technical factors related to the performance and success of CSE.(66–68) Although CSE is considered a relatively new technique, in 1937 Soresi actually described the intentional injection of anesthetic agents outside and within the subarachnoid space.(69) Somewhat different from current practice, Soresi intentionally used a single needle. He first injected some local anesthetic into the epidural space and then advanced the needle and injected the rest of the medication to cause a subarachnoid block. Although this technique included both spinal and epidural anesthesia, no catheter was used.
In 1979, Curelaru(70) reported the first CSE with an introduction of an epidural catheter through a Tuohy needle. Catheter insertion was followed by a test dose and then a traditional dural puncture, which was performed at a different interspace using a 26-gauge spinal needle. That same year, Brownridge suggested the use of CSE for obstetrics. He described successful use of CSE for elective cesarean section in 1981.(71,72)
In 1982, the NTN CSE technique was first described independently by Coates and Mumtaz, and its active use in obstetric practice was first published in 1984 by Carrie.(73–75) Popularity of the technique began in the late 1990s. Several approaches for Initiation of CSE have been described in the recent literature.
In contrast to Soresi’s initial description of CSE, in which a single needle was introduced into the epidural space and then advanced into the subarachnoid space, the currently preferred NTN technique includes use of separate epidural and spinal needles. Typically, the epidural space is located with a conventional epidural needle and technique, and then a long spinal needle is passed through the epidural needle until CSF appears in the hub of the spinal needle. The drug is administered via the spinal needle into the subarachnoid space, the spinal needle is removed, and finally an epidural catheter is inserted into the epidural space. Although several different CSE techniques are used in clinical practice (including the two-needle, two-interspace technique), NTN is the most widely used CSE technique in the United States.
The CSE technique may be performed using two separate needles with the separate needle technique (SNT), with spinal block and epidural catheter placement at either a single (76,77) or two different interspaces. (78–80) If the epidural catheter is placed first, proper placement can be tested before administration of spinal medications, potentially decreasing the risk of accidental intravascular or intrathecal catheter migration. Placing the epidural catheter first may also reduce the risk of neural damage, which may occur when the catheter is inserted after subarachnoid block, because paresthesia and other warning signs of improper needle placement may be absent after administration of spinal medications. However, there is also the risk of striking the epidural catheter with the spinal needle.(81–83) Some authors consider this to be a purely hypothetical risk and have demonstrated that it is not possible to perforate an epidural catheter with commonly used spinal needles.(84,85)
Cook et al (86) reported a series of 201 consecutive CSEs performed with a novel SNT. The study was designed to avoid potential and actual problems associated with the CSE technique. Cook et al placed the spinal needle in the subarachnoid space and then replaced the spinal needle stylet to stop the CSF leak. Next, the epidural catheter was placed through a different interspace and then returned to the spinal needle to inject the subarachnoid drug, thus avoiding epidural catheter insertion in an anesthetized patient. This method of CSE anesthesia, although much more work, may be associated with high success and low complication rates.
Regardless of which component is performed first, the major disadvantage of the two-needle, two-interspace technique is that it takes longer to perform and requires two separate injections.
The SNT technique has a few theoretical advantages compared to the NTN technique. It enables placement of the epidural catheter prior to initiation of the spinal block. The SNT may thus theoretically reduce the risk for neurological injury because paresthesia and other symptoms are not masked. Because the epidural catheter is placed early, problems that may occur due to delayed catheter placement (technical problems) after the injection of a hyperbaric spinal solution (such as unilateral, sacral, or low lumbar regional neuraxial block) are avoided.(87–89)
Several studies have compared NTN and SNT techniques.(90–93) Some have reported better success and lower failure rates with the SNT. However, these studies also reported greater patient acceptance and less discomfort with the NTN technique. Backe et al, in a prospective randomized study,(94) compared the outcomes and techniques of NTN and SNT (double space) CSE in 200 elective cesarean delivery patients. Successful blocks to T5 with the double-space and the NTN techniques were 80 versus 54, respectively, odds ratio 0.29. SNT had a greater success rate than the NTN technique; the T5 dermatome was reached with fewer corrective manipulations (epidural augmentation or repeated blocks). Failure to enter the intrathecal space once the epidural space had been located occurred in 29 patients in the NTN group. Time to readiness for surgery, however, was slightly increased with SNT (15 minutes with SNT vs. 12.9 minutes with NTN).
Sadashivaiah et al (95) retrospectively analyzed data from 3519 elective cesarean deliveries performed under the SNT technique. They reported a lower rate of conversion to general anesthetic due to failed neuraxial block (0.23%) than previously reported (0.8%–1.3%).
One of the problems with the NTN technique is that many patients complain of paresthesia/dysesthesia or respond (movement, grimacing, vocalization) to dural puncture during insertion of a pencil-point needle. Van den Berg et al (96) compared the effects of saline versus air for loss of resistance (LOR) on the occurrence of this discomfort and reported that use of saline is associated with fewer patient (18% vs. 44%) responses at the moment of thecal penetration. Although the mechanism of this reduced response with saline for LOR is not clear, the authors postulated that perhaps the placement of saline in the epidural space modulated dural sensitivity.
The success of a CSE block is heavily dependent on accurate cannulation of the epidural space. The identification of the epidural space is traditionally achieved by a blind LOR technique. With this handling of the needles, where the feedback to the operator is merely tactile, deviation of the axis of the needle trajectory may occur. Because of the triangular form of the dural sac, deviation of the spinal needle from the midline will cause the dural sac to be missed, leading to spinal component failure or unsuccessful dural puncture.
Grau et al performed real-time ultrasound scanning of the lumbar spine to provide an accurate reading of the location of the needle tip and to facilitate the performance of CSE anesthesia.(97) Their aim was to establish a less-invasive method to monitor the advancement of the needle in real-time. Thirty parturients scheduled for cesarean delivery were randomized to three equal groups. Ten control patients received CSE anesthesia performed in a conventional manner. Ten received ultrasound scans by an off-line technique. The remaining 10 received online imaging of the lumbar region during puncture. The Tuohy needle was inserted using the midline approach in all three groups. In the control group, CSE was performed using A single-space NTN technique with the standard LOR to saline method.
In the off-line group, ultrasound images were taken just before the puncture to improve needle trajectory. In the online group, ultrasonic images were taken to monitor and identify needle trajectory in real-time.
The authors reported that in both ultrasound groups, a significant reduction in the number of necessary puncture attempts was found (p < .036); the number of interspaces necessary for puncture was reduced (p < .036); and the number of spinal needle manipulations was significantly reduced (p < .036). Dural tenting was observed in 9 of 10 of the online group (tenting length 2.4 mm). Asymmetric block was observed in 10% of those in the control group, but not in any of those in the ultrasound groups. The authors concluded that the use of ultrasound imaging was obviously helpful in finding the ideal needle trajectory and to improve puncture conditions by the demonstration of the relevant anatomy.
In the CSE NTN technique, there is no practical test to confirm correct epidural catheter placement. Tsui and colleagues proposed the use of nerve stimulators to confirm the proper placement of epidural catheter.(98) They studied 39 obstetric patients in labor, receiving epidural catheters (not CSE) for analgesia. A low-current (1- to 10-mA) electrical stimulation was used to confirm the correct placement of the epidural catheter (19-gauge Arrow Flextip plus). A positive motor response (truncal or limb) indicated that the catheter was in the epidural space. They reported that the sensitivity and specificity of this test were 100% and 100%, respectively, with 38 true positive tests and 1 true negative test. A case of intravascular epidural catheter migration was detected using this new test and was subsequently confirmed by a positive epinephrine test. If the motor response only occurs with larger currents (>10 mA) or does not respond at all (before receiving any local anesthetics), the catheter is most likely outside the epidural space. If a positive response occurs at an unusually low milliamperage (<1 mA), intrathecal placement is likely.
The electrical stimulation test may not be applicable when the CSE technique is used for surgery, where anesthetic doses of local anesthetics are administered intrathecally prior to the placement of the epidural catheter. When using the CSE technique for labor analgesia, this test may be utilized as a simple and practical method to determine the epidural catheter placement. The standard test dose utilized in the United States (3 mL of 1.5% lidocaine with 1:200,000 epinephrine) may help to identify intravascular and intrathecal placement, but it does not verify appropriate epidural placement or function.
/Chapter 19: CSE/CH19_CSE - Choice of LA.jpg)
Sufentanil and fentanyl, with or without local anesthetics, are most often administered intrathecally to provide analgesia for the laboring woman receiving CSE. The usual doses of sufentanil are 2.5–10 μg; however, most practitioners are now using 2.5 or 5 μg. The ED50 and ED95 for laboring patients were found to be 2.6 and 8.9 μg respectively.(99) The doses of fentanyl used are typically10–25 μg. The median effective dose (ED50) and effective dose in 95% of the population (ED95) for laboring patients have been reported to be 5.5 and 17.4 μg, respectively.(100) Although the original studies used much higher doses of intrathecal opioids (10 μg sufentanil and 25–50 μg of fentanyl), subsequent studies have suggested the use of smaller doses, with reduced side effects and similar analgesic effect.(101)
Morphine, a highly ionized, water-soluble opioid, produces analgesia of long duration but slow onset (approximately 60 minutes between neuraxial injection and onset). In addition, it may be associated with an unacceptably high incidence of side effects, such as nausea, vomiting, pruritus, as well as the potential for delayed respiratory depression. These side effects, coupled with the slow onset of pain relief, limit the usefulness of intrathecal morphine for labor analgesia. Intrathecal meperidine (10 mg) may provide reliable analgesia in advanced labor102 but has been associated with a high incidence of nausea, vomiting, hypotension, and need for low blood pressure management. In addition, it is the only opioid that has intrinsic local anesthetic properties at clinically appropriate doses(102) by blocking nerve conduction at the proximal end of the dorsal root(103) via a mechanism other than sodium channel block.(104) This nerve conduction block is not reversible with naloxone.(103)
In many patients, a single intrathecal injection of a lipid-soluble opioid is insufficient to produce analgesia for the entire duration of labor. If the second stage of labor is imminent, to achieve a greater depth of pain relief, the subarachnoid administration of local anesthetic plus opioid should be considered.
The combination of 2.5–5 μg sufentanil plus 2.5 mg bupivacaine provides rapid analgesia without motor block, alleviates the pain of the second stage of labor, and lasts longer than sufentanil alone.(105) Although the original reports(106) recommended the use of 10 μg of sufentanil, Sia and colleagues showed that adequate labor pain relief could be safely provided by administering half that dose of intrathecal sufentanil plus bupivacaine.(107)
Previous studies(108) have attempted to determine the ED50 of intrathecal bupivacaine, defined as minimum local anesthetic dose (MLAD) or ED50 and then use this to assess the effect of different doses of fentanyl. The MLAD of intrathecal bupivacaine has been found to be 1.99 mg, and the addition of 5 μg intrathecal fentanyl offered a similar significant sparing effect to 15 or 25 μg of fentanyl, resulting in less pruritus but with a shortened duration of action. ED95 was estimated from those studies.
Whitty et al(109) performed an up-down dose-finding study to determine the ED95 for intrathecal bupivacaine (more clinically relevant than that calculated from ED50) when combined with a fixed amount of fentanyl. They recommended 1.75 mg of bupivacaine with 15 μg of fentanyl to reliably and rapidly relieve pain of parturients in the active phase of labor. At Jackson Memorial Hospital (Miami, FL), we currently use 1.25 mg bupivacaine plus 15 μg fentanyl as our spinal drug.
Levin et al compared a standard dose of intrathecal bupivacaine with sufentanil for CSE analgesia using two doses of ropivacaine (2 and 4 mg) with sufentanil. They concluded that both local anesthetics provided similar labor analgesia duration with equivalent side effects.(110)
/Chapter 19: CSE/CH19_CSE - Complications and Side Effects.jpg)
The most common method of performing a CSE is the single interspace NTN technique. Failure to achieve a spinal block with this technique has been reported in 10%–15% of cases in the past,(111,112) although in experienced hands this risk may be as low as 2%–5%.(113)
Possible causes for failure of CSE include the following:
Most current needle designs allow extension of the spinal needle 12–15 mm beyond the tip of the Tuohy needle. Excessively long needles, however, pose problems of handling and depth of placement. Deviation from midline will lengthen the epidural-dural distance and may also cause the spinal needle to miss the spinal space laterally (Figures 1 and 2). In addition, preservative-free normal saline used to identify the epidural space may be misinterpreted as CSF.



One of the concerns with the CSE technique is that the epidural catheter may unintentionally pass through the dural puncture hole into the subarachnoid space during the CSE technique. This seems more likely with the NTN CSE technique than the SNT or with epidural needles with back holes (Figure 3). Although this may seem a rare theoretical problem, several publications have reported its occurrence.(126–129)

Angle et al (130) studied factors contributing to unintentional subarachnoid catheter passage after epidural placement with an in vitro model using human dural tissue. In that study, the dura was punctured with 25-gauge Whitacre® spinal needles. The likelihood of the catheter to enter into the subarachnoidal space was compared between the intact dura, versus the dura with obvious epidural needle punctures, and single 25-gauge.
Whitacre spinal needle punctures after a CSE technique. Their conclusion was that the catheter passage is unlikely to occur in the presence of an intact dura or after an uncomplicated CSE technique. Therefore, unintentional subarachnoid passage of the epidural catheter suggests dural damage with the epidural needle.
Holtz et al investigated the possible passage of the epidural catheter into the subarachnoid space in an anatomical preparation.(131) In 10 series of experiments, the epidural compartment was entered with an 18-gauge Tuohy needle. The spinal puncture (27- or 29- gauge Quincke needle) was performed with the NTN technique. Subsequently, the internal side of the intrathecal compartment was examined endoscopically for penetration of the epidural catheter. In a similar way, the endoscope was inserted epidurally to visualize the movements of the epidural catheter in the epidural compartment. In this model of simulated physiologic intrathecal conditions, using one space NTN technique, they could not detect intrathecal passage of the epidural catheter.
Holmstrom and colleagues, in a percutaneous epiduroscopy study using fresh cadavers, also reported that it was impossible to force an epidural catheter into the subarachnoid space after a single perforation of the dura with a small-gauge spinal needle. However, they found that the risk of intrathecal catheter migration increased to approximately 5% after multiple dural punctures with the spinal needle. Dural penetration of the epidural catheter after a dural puncture with a Tuohy needle was clearly demonstrated in the same study.(132)
Whether the incidence of an unintentional passage of the epidural catheter into the subarachnoid space is increased with CSE as compared to standard epidural technique alone is controversial. Therefore, regardless of technique used, all epidural medications should be given in incremental doses.
Leighton and colleagues reported that, following a CSE, a dose of epidural local anesthetic will produce a higher dermatomal level than expected, presumably due to subarachnoid flux of the drug.(133) However, when used for labor analgesia, unless the dura is breached with the epidural needle or large bolus volumes are administered,(134) flux should not be clinically significant. Suzuki et al found, in nonpregnant patients, that dural puncture using a 26-gauge Whitacre spinal needle before the epidural injection increased caudal spread of analgesia induced by epidural local anesthetics with no change in the cephalad spread.(135)
Holtz et al endoscopically investigated the possible passage of epidural anesthetic through the dural puncture hole into the CSF compartment in an anatomical preparation.(131) Even 1 hour after epidural administration of 20 mL of methylene blue–dyed local anesthetic (bupivacaine 0.5%, isobaric), no passage of local anesthetic into the intrathecal compartment could be detected under continuous endoscopic monitoring.
A study by Kamiya et al(136) measured the lidocaine concentration in CSF after epidural administration at different interspaces with or without preceding spinal anesthesia. They concluded that there was no difference in the lidocaine concentrations in CSF with or without a meningeal hole. The authors explained the possible reason for the lack of difference in lidocaine concentration as follows: Lidocaine readily penetrates through meningeal tissue, and this transfer efficiency was most likely not affected by the presence of a small meningeal hole. The equilibrium of the lidocaine concentration in CSF, close to the administration site, would be attained within a few minutes due to this rapid penetration. Stated differently, the amount of local anesthetic that crosses through the small hole in the dura is trivial when compared with the amount that crosses through the meninges. This study confirmed that CSE is safe, and that the dural holes have no clinically significant influence on duration or extent of the spinal blocks in patients undergoing cesarean delivery. The data from several clinical studies of the CSE technique have not indicated an increase in spread of sensory block due to subarachnoid leakage of epidurally administered medications.(131,137–139)
However, the magnitude of flux is a function of the diameter of the spinal needle, and the risk may be increased by using a larger spinal needle or in the presence of a hole made with a Tuohy needle. The possibility of this hazard is supported by reports of high or total spinal block during epidural anesthesia administered following unintentional dural perforation with the epidural needle.(140,141)
The administration of a test dose for spinal placement of an epidural catheter may be problematic and aspiration may fail, but test doses have been found to detect more intrathecal catheters than aspiration alone during labor analgesia.(142) Despite studies that have reported that intrathecal migration is very rare and that the flux should not produce clinically relevant complications, the reader is cautioned that epidural drugs or catheters may migrate into the spinal space following CSE. Therefore, all epidural doses should be incremental, and patients receiving continuous epidural infusions for analgesia should be checked approximately every hour to rule out excessive motor or sensory block that may be indicative of unintentional intrathecal administration of drugs.
Does subarachnoid block induced by CSE (using LOR to air) render a higher level of sensory anesthesia than SSS when an identical mass of intrathecal anesthetic was injected? Goy et al performed a prospective randomized study comparing CSE (using LOR to air) versus SSS on 60 patients who were undergoing minor gynecological procedures and concluded that subarachnoid block induced by CSE produces greater sensorimotor anesthesia (p < .01) and prolonged recovery (p < .05) than SSS.(143) They also found a more frequent incidence of hypotension and vasopressor use in the CSE group (p < .05), despite using identical doses of intrathecal medications.(143)
Another study reported similar findings when only 4 mL of air were used as part of the LOR technique.(144) The objective of that study was to determine the ED50 of intrathecal hyperbaric bupivacaine for CSE and SSS by using the up-down sequential allocation technique. Sixty participants were allocated into two groups in a double-blind, randomized, prospective study design. They concluded that, under similar clinical conditions, the ED50 of intrathecal hyperbaric bupivacaine in CSE was 20% less than that in SSS. Although the mechanism that accounts for this finding has not been determined, one possible explanation is that the LOR to air technique in CSE could introduce air pockets within the epidural space. MRI has demonstrated residual air pockets to extend up to three lumbar vertebral segments and compress the lumbar thecal sac dorsally and laterally.(145) This could potentially result in a reduction of the lumbosacral CSF volume and enhance the extent of sensory anesthesia.(146)
Epidural administration of drugs seems to affect the thecal contents and therefore influence the spread of earlier induced subarachnoid block.(147,148) The magnitude of this effect depends on the time interval between the injections and the volume of the epidural injectate. Initially, the proposed mechanism for this effect was the subarachnoid leak of epidurally administered medications.
Hypotension can occur following the administration of intrathecal fentanyl or sufentanil, even if sympathetic block does not occur. However, the hemodynamic effects of intrathecal fentanyl are usually benign in nature and may actually be due to a decrease in catecholamines secondary to pain relief. Vasodilation due to sympathectomy, however, causes a decrease in preload, end-diastolic index, and stroke index, and an increase in heart rate. Because the end-diastolic index and stroke index remained relatively stable and the heart rate decreased in a study by Mandell and colleagues, these authors concluded that the observed hypotension was not due to vasodilation.(149) The hypotensive episodes following administration of neuraxial opioids for labor are transient, easily treated, and not necessarily associated with adverse fetal heart rate changes.
Neurological complications directly related to spinal anesthesia may be caused by trauma, cord ischemia, infection, and neurotoxicity.
Needle- or catheter-induced trauma rarely results in permanent neurological injury. However, Horlocker et al, in a retrospective review of 4767 consecutive spinal anesthetics for central nervous system complications, concluded that the presence of a paresthesia during needle placement significantly increased the risk of persistent paresthesia (p < .001). In that review, paresthesia was elicited during needle placement in 298 (6.3%) cases. Six patients reported pain (persistent paresthesia) on resolution of the spinal anesthetic; four of these individuals had pain that resolved within 1 week, and the pain of the remaining two resolved in 18–24 months.(150) According to a more recent study by Bigeleisen on peripheral nerve block, nerve puncture and intraneural injection did not invariably lead to neurological injury.(151)
There are a few reasons for a possible increase in the risk of neurological sequelae following the CSE technique. In the single-space, NTN technique of CSE, the insertion of the epidural needle and catheter after administration of spinal local anesthetics may prevent identification of paresthesias that may warn the anesthesiologist about needle misplacement. Higher incidence of paresthesia during CSE is a recognized factor. In fact, it has been reported that paresthesias occur in up to 11% of patients undergoing CSE.(152) Browne et al reported a 14% incidence of paresthesias with the Espocan needle (18-gauge Tuohy epidural needle with an extra lumen in the needle bevel) and a 42% incidence with a conventional Tuohy epidural needle.(15)
In a randomized prospective study, McAndrew et al similarly reported that 37% (17 of 46) of women in the NTN CSE group and only 9% (4 of 43) in the SSS group had paresthesia on spinal needle insertion (p < 0.05). The equipment used was a 16-gauge/26-gauge CSE kit and a 26-gauge pencil-point spinal needle with introducer (both Sims Portex, Australia). They postulated that the higher incidence of paresthesias may be related to deeper penetration of the subarachnoid space with the CSE technique. Interestingly, in that study, none of the patients had persistent neurological symptoms on examination at postoperative day 1.(153)
Holloway et al conducted a pilot survey of anesthetists’ experiences of neurological sequelae following spinal and CSE anesthesia in the obstetric units in the United Kingdom.(154) Because of the retrospective nature of the survey, many neurological problems that were reported lacked detail. However, there were no obvious differences in incidence of problems associated with CSE versus the SSS techniques.
Turner and Shaw suggested the possibility that painful insertion and subsequent root damage might be increased by the use of atraumatic pencil-point spinal needles.(155) In that survey, problems were reported with both Whitacre and Sprotte needles, but none with Quincke needles. However, the numbers using Quincke needles were too small to allow statistical analysis.
More dangerous than root damage is damage to the spinal cord itself, and in that survey,(155) there were two cases of conus damage, one with CSE and one with SSS. This complication is not a fault of atraumatic needles, but rather of the technique. It is important to remember that in 19% of patients, the spinal cord terminates below L1. Even more worrisome, in more than 50% of cases, the chosen space is incorrectly identified.(156) Therefore, a space L3/L4 or below should be selected for CSE or SSS.
It has been alleged that during the NTN CSE technique, tiny metal particles abraded by the spinal needle from the inner edge of the Tuohy needle may be introduced into the epidural or spinal compartment.(157) To examine this concern, Holst and colleagues simulated the NTN technique in an in vitro model.(131) They used atomic absorption spectrography (AAS) to identify abraded metal particles. The needles were then examined under an electron microscope. They reported no increased alloy components detected in the rinse solution after either twofold or fivefold puncture compared with the control measurements. After five punctures and handling the needle as in normal practice, no traces of use could be detected by electron microscopy on the inner ground edge of the Tuohy needle.(131)
Tissue coring is a phenomenon that may occur during lumbar puncture, in which pieces of tissue are removed by the needle as it passes through the tissue and deposits the pieces in the subarachnoid space. Although rare, adverse outcomes such as intraspinal iatrogenic epidermoid tumors may be associated with this phenomenon. Sharma et al(158) postulated that the CSE technique introduces fewer epithelial cells in the subarachnoid space when compared with the SSS without the use of an introducer. However, this study did not support the hypothesis. Significant tissue coring occurred with both techniques (CSE 88% and SSS 96%).
Although overall incidence of infections and their sequelae following placement of CSE is perceived to be extremely low, the relative risk compared to either a spinal or epidural techniques alone is not known. In a classic study, Dripps and Vandam prospectively reported no cases of meningitis after 10,098 spinal anesthetics.(159) Phillips et al also reported no cases after a prospective review of 10,440 such cases.(160) These studies included patients undergoing obstetric and urological operations, which are known to be associated with perioperative bacteremia. However, case reports of meningitis following CSEs appeared in the journals beginning mid 1990s.(161,162)
Theoretically, CSE is thought to be associated with an increased risk of meningitis compared to epidural alone because the dura (protective barrier for the central nervous system) is punctured deliberately during CSE, and then a foreign body, an epidural catheter, is placed nearby. The epidural catheter can lie close to the dural hole and is a potential focus of infection, especially following bacteremia.(163) Contamination of the subarachnoid space may occur from bleeding due to needle trauma in a bacteremic patient or from failure of aseptic technique.
Several studies have shown that face masks prevent forward dispersal of organisms from the upper airway and downward dispersal during talking and head turning.(164,165) Despite this, in 1996 a postal survey of members of the Obstetric Anaesthetists Association in the United Kingdom found that over half those surveyed did not routinely wear face masks when performing neuraxial anesthesia.(166)
In 2007, for the first time, the Healthcare Infection Control Practices Advisory Committee (HICPAC) recommended that surgical masks be worn during spinal procedures to prevent infections.(167) This recommendation was made in response to several reports of meningitis following myelography procedures. In 2008, three bacterial meningitis cases in postpartum women were reported to the New York State Department of Health. All three women received CSE for labor. Streptococcus salivarius (a normal commensal of oral flora) was cultured from the CSF of two patients. The anesthesiologist responsible for all three cases reported routine use of masks during neuraxial procedures. However, the staff reported that it was common to have unmasked visitors present in the room during these procedures. The hospital instituted new policies to minimize visitors and to require masks for all persons in the room during neuraxial labor analgesia procedures. In 2009, two similar cases were reported to the Ohio Department of Health. The anesthesiologist responsible for these two cases did not wear a mask. CSF cultures from both patients revealed S. salivarius, and one of them died from suppurative meningoencephalitis.(168) In 2009, Sankovsky et al(169) also reported a case of S. salivarius meningitis subsequent to CSE for labor in a healthy primigravid patient. The anesthesiologist was wearing sterile gloves and a mask, but the mask had been worn during prior procedures.
These cases highlight the importance of adhering to established infection control recommendations during neuraxial procedures, which include the use of masks, washing hands, and adherence to aseptic technique. It is important that the face mask be tightly attached to cover the mouth and nose and not be reused.
Headache and neck pain or neck stiffness in a patient who recently received spinal anesthesia is often attributed to postdural puncture headache (PDPH). One case report highlighted the dangers associated with missed diagnosis of meningitis. The patient was misdiagnosed as having endometritis when presenting with headache, vomiting, and fever for 2 days after uncomplicated epidural analgesia for labor. Her condition rapidly deteriorated, and meningitis was not considered as a diagnosis until it was too late. She subsequently died in intensive care.(170)
Hyperbaric bupivacaine is frequently administered intrathecally during CSE anesthesia. Although neurological problems are mostly reported following administration of lidocaine or mepivacaine, a few cases of cauda equina syndrome following ordinary doses of intrathecal bupivacaine in a CSE technique have been reported.
Tariq (171) reported a case of an 83-year-old man who developed cauda equina syndrome after uneventful CSE anesthesia for elective knee arthroplasty. Takasu et al(172) reported a 29-year-old parturient who developed cauda equina syndrome following uneventful CSE with hyperbaric bupivacaine for cesarean delivery. Kubina et al(173) also described two cases of cauda equina following uneventful CSE with hyperbaric bupivacaine. One of the patients, however, suffered from spinal stenosis, which could explain this complication. Kato et al described a case of cauda equina syndrome following CSE with an ordinary dose of hyperbaric bupivacaine in an older patient without spinal stenosis.(174) It is thought that the lack of a protective sheath in the cauda equina as the spinal nerves and roots pass through the dura makes them particularly prone to injury from high concentration of local anesthetics.
The incidence of PDPH after CSE technique is controversial; some authors have reported decreased incidence when compared with epidural technique alone,(175) while others report an increased incidence.(176) Balestrieri reported that the patients who received conventional epidural analgesia were more likely to suffer an accidental dural puncture (twofold increase; epidural vs. CSE = 4.2% vs. 1.7%). They offered two possible explanations for this result. The first reason was that they usually chose CSE for women who were most often in early labor and reserved epidural analgesia for patients in the more painful active phase of labor. Therefore, the patients in the epidural group were more likely to move during the procedure and thus cause a “wet tap.” Second, during CSE if uncertain of the location of the epidural needle, the spinal needle could be inserted to look for CSF(177) and the epidural needle advanced no further after seeing CSF in the spinal needle.
There are other factors that may also decrease the incidence of PDPH following the CSE technique. Administration of intrathecal opioids has been shown to decrease the incidence of PDPH.(178) Subsequent infusion of epidural local anesthetic increases the subarachnoid pressure and may help to decrease the incidence of PDPH following CSE. Dunn et al. argued that the intentional dural puncture involved in the CSE technique would increase the risk of PDPH in obstetric patients compared to epidural analgesia alone.(176) The use of small-gauge atraumatic pencil-point spinal needles (such as Whitacre, Pencan, Sprotte, and Gertie Marx) will greatly reduce the incidence of PDPH in patients receiving CSE.(175,179)
Chan and Paech reported three cases of persistent CSF leak following uneventful CSE analgesia for labor.(180) It was confirmed that the leaking fluid was CSF in two cases by β2-transferrin immunofixation assay.(181) None of the patients developed PDPH or any other complications. Howes and Lenz also reported a CSF cutaneous fistula in two patients following epidural anesthesia (not CSE) for postoperative pain relief. Both patients developed PDPH only after removal of the catheters and were treated successfully with autologous blood patch.(182)
Reports in the literature suggested an increased frequency of nonreassuring fetal heart rate (FHR) tracings and fetal bradycardia associated with CSE.(183–185) The etiology of fetal bradycardia after CSE remains elusive but may be related to an acute reduction in circulating maternal catecholamine levels after the almost-immediate onset of analgesia. In addition, it has been postulated that an imbalance between epinephrine and norepinephrine levels (decreased epinephrine levels in the continuing presence of high norepinephrine levels) causes unopposed α-adrenoceptor effects on uterine tone with increased uterine vascular resistance leading to decreased uterine blood flow.
A meta-analysis by Mardirosoff and colleagues found a relative risk of 1.81 of having FHR abnormalities when intrathecal opioids were used. However, the risk of subsequent cesarean delivery was not increased.(186) There is evidence of a dose relationship and greater occurrence of worrisome FHR abnormalities with higher doses of opioids.(187) Nicolet et al performed a prospective study to identify maternal factors implicated in fetal bradycardia after CSE for labor pain. They found that the level of maternal pain scores at the time of labor analgesia request and maternal age were independent predictors of fetal bradycardia after neuraxial analgesia for labor.(188)
The resulting fetal bradycardia was usually short-lived and typically resolved within 5–8 minutes.(189) A retrospective study of 1240 patients who received regional labor analgesia (mostly CSE) and 1140 patients who received systemic medication or no analgesia demonstrated no significant difference in the rate of cesarean delivery, with rates of 1.3% and 1.4%, respectively. That study also reported that no emergency cesarean deliveries for acute fetal “distress” were necessary in the absence of obstetric indications up to 90 minutes after intrathecal sufentanil administration.(190) A prospective randomized study by Skupski et al(191) also found no difference in the rate of prolonged deceleration between labor epidural versus CSE for labor (3.2% vs. 6.2%, respectively; p = 0.43).
The CSE technique has gained popularity and acceptance, especially in obstetrics. Special kits have been produced for CSE (eg, B Braun Medical Ltd. comprising the standard 16-gauge, 8-cm Tuohy needle with a 26-gauge Quincke spinal needle). Various concerns of the CSE technique have led to some modification of the needles used.
To direct the epidural catheter away from the dural puncture site, Rawal et al recommended rotation of the epidural needle 180° following dural puncture. This maneuver directs the epidural catheter 2–2.5 mm away from the dural puncture site.(192) However, Meikljohn, using postmortem dura mater, demonstrated that rotation of the epidural needle significantly decreased the force required to puncture the dura(117) and thus might result in a wet tap.
Recently, CSE kits designed with an orifice in the back curve (back hole) of the epidural needle for separate spinal needle passage have been made available(15) (Figure 4). This needle and others like it may reduce the likelihood of dural passage of the epidural catheter by directing the catheter away from the dural puncture site. However, the spinal needle may not always go through the spinal needle orifice and may exit through the Huber tip, thus losing the advantage of the back hole(15) (Figure 5).



Joshi and McCarroll suggested a technique to enhance the spinal needle exit through the spinal needle orifice.(111,193) The modified technique consisted of first aligning the bevel orifice of the spinal needle to the same direction as the Tuohy bevel and then bending the spinal needle 10° toward the Tuohy bevel while advancing through the Tuohy needle. This technique guides the spinal needle tip to exit through the back hole. Pan, in a prospective randomized study, evaluated the success rate of the spinal needle existing through the Spinal needle orifice in two commonly available single-lumen, dual-orifice, CSE needle kits.(194) The CSE kits studies were first the Espocan CSE kit (Braun Medical Ltd.) that consists of a standard 18-gauge Tuohy needle with a 26-gauge sleeved Quincke spinal needle that extends 12 mm beyond the tip of the Tuohy needle through the back hole. The sleeve on the spinal needle was designed to guide the spinal needle to exit through the back hole. Second was the Espocan CSE kit (Braun Medical Ltd.), which consists of the same epidural needle with a 27-gauge nonsleeved Sprotte spinal needle that extends 13 mm beyond the tip of the Tuohy needle through the back hole. They performed 1600 attempts, which included the modified technique described by Joshi and McCarroll. The modified technique improved the success rate of spinal needle exiting through the back hole from 67% to 94% for the first kit and 50% to 81% for the second kit; cephalad orientation of the Tuohy needle bevel further improved the success rate to 96% and 91%, respectively. Overall, the sleeved spinal needle had a better success rate than the unsleeved spinal needle.
The failure of the spinal needle to exit through the back hole may also result in bending of the spinal needle and less protrusion beyond the tip of the Tuohy needle. This may contribute to the increased failure rate of dural puncture. The ideal length of spinal needle protrusion is reported to be at least 12–13 mm. In a prospective randomized study of 40 patients, Joshi and McCarroll reported a 15% failure rate of CSF return when the spinal needle protruded only 10 mm beyond the tip of the Tuohy needle and 0% with a 13-mm protrusion.(111) Riley et al reported(195) similar results comparing 24-gauge Sprotte (9-mm protrusion past the tip of the Tuhoy and 17% failure to obtain CSF) and Gertie Marx (protrusion 17 mm and 0% failure rate). The number of patients developing PDPH and requiring blood patch was greater with Gertie Marx than the Sprotte needle. However, this difference was not statistically significant. It is possible that the longer spinal needle also punctured the anterior aspect of the dura and thus might have caused a greater CSF leak. Greater rates of paresthesia were also noted (anecdotal) with the 127-mm needle, and the 124-mm Gertie Marx needle was suggested as an excellent compromise.
Herbstman et al compared four pencil-point spinal needles commonly used in the CSE technique and reported that longer spinal needles were associated with significantly more transient paresthesias (Gertie Marx 15-mm protrusion with 29% incidence; Whitacre 10-mm protrusion with 17% incidence). Success in obtaining CSF and the incidence of PDPH did not differ among the four needles.(196)
The conventional spinal needle in the CSE kit, which does not lock within the epidural needle, may be difficult to handle and stabilize during injection of spinal medication. The displacement of the spinal needle during aspiration of the CSF and injection may result in failed anesthesia or may push the spinal needle deeper, leading to nerve damage or anterior dural perforation. To overcome this problem, Simsa suggested an external fixation device.(197) This device, however, is somewhat complicated to handle.
Recently, spinal needles with an adjustable locking device have been introduced (CSEcure and Adjustable Durasafe CSE needle). Studies of the lockable extensions reported them to provide safe and stable conditions during placement of the syringe and injection.(62,198) However, both studies reported frequent inability to feel dural perforation with the locking needles (15.3% with CSFcure and 25% with Adjustable Durasafe). There was no clear explanation for this.
In a CSE technique, sometimes the epidural catheter cannot be threaded or threaded intravascularly after the intrathecal drugs have been injected. To overcome this problem, a dual lumen, dual-orifice CSE kit was developed in which an epidural catheter can be inserted in place prior to inserting the spinal needle and medication.(199,200) This is possible because there are two separate lumens for the catheter and the spinal needle (Figure 6).

Recently, a dual-lumen CSE needle was commercialized in Europe (Epistar; Medimex, Germany).
The issue of whether a test dose is needed when administering labor epidural analgesia is controversial.(201,202) Because very dilute solutions of LAs are commonly used and aspiration is often diagnostic, some authors believe that a conventional test dose is unnecessary.(203) However, because catheter aspiration is not always predictive (especially when using a single-orifice epidural catheter), others maintain the importance of a test dose to improve detection of intrathecal or intravascular placement of an epidural catheter.(204)
Part of the controversy surrounding the testing of epidural catheters involves the use of epinephrine. Epinephrine has been shown to produce a reliable increase in heart rate in volunteers and surgical patients when the epidural has been sited in a blood vessel.(204) However, in laboring women, maternal heart rate variability from the pain of uterine contractions may confuse interpretation of the heart rate response, and intravenous epinephrine may have deleterious effects on uterine blood flow.(205)
Means to improve the reliability of an epinephrine test dose include injecting the dose between uterine contractions and repeating the test dose when the response is equivocal. However, the lack of sensitivity and specificity of the test dose calls into question its usefulness as a diagnostic tool.
Leighton and colleagues have described an alternative means of testing an epidural catheter for intravascular placement. They advocated the injection of 1–2 mL of air into the epidural catheter while listening over the precordium with the maternal external Doppler monitor for evidence of air.(206)
With reports of subarachnoid administration of chloroprocaine,(207) it is possible that in the future this agent will be utilized for testing epidural catheters. Caution, however, is necessary because preservative-containing chloroprocaine is also commercially available. If continuous infusion of dilute local anesthetic is administered and the patient remains comfortable without a motor block, proper epidural catheter placement is highly likely. That is, if the epidural catheter were intravascular, the patient should have inadequate pain relief, and if the catheter were subarachnoid, a solid motor block would develop. Although infusions of ultra dilute local anesthetics do not pose a serious threat, such is not true of concentrated local anesthetics used for operative delivery. Some authors have suggested that a test dose is essential for any parturient receiving epidural anesthesia.(204) Regardless of the technique used, the safe practice of administering labor epidural analgesia dictates initial catheter aspiration, incremental injections, and continuous monitoring for evidence of local anesthetic toxicity.
Neuraxial blocks are often performed with the patients in the sitting position, especially obese individuals, because the midline is easily recognized. The sitting position has been shown to allow better spinal flexion in the parturients.208 In addition, the distance from the skin to the epidural space was shown to be significantly greater when epidural puncture was performed in the lateral position as compared with the sitting position. This change in distance may cause catheter dislodgment when the patient is turned from the sitting position to lateral, with consequent inadequate analgesia.
Yun et al compared the effects of induction of CSE anesthesia in the sitting versus lateral position in healthy women undergoing elective cesarean delivery.(209) The severity of hypotension, measured by the maximal percentage decrease in systolic blood pressure from control, as well as its duration were significantly greater in the sitting group (p < 0.05). Patients in the sitting group required twice as much ephedrine to treat hypotension than those in the lateral recumbent group. The reason for the difference in the severity of hypotension was not clear. They postulated it to be related to a slower recovery from venous pooling in the lower extremities when assuming a supine position from the initial sitting position. These authors concluded that the position used for induction of CSE should be considered in cases associated with greater maternal or fetal risk from hypotension.
Traditional teaching is that the spread of hyperbaric intrathecal solutions follows gravity. Lewis et al compared the development of spinal blocks in the left lateral position versus a supine wedge position after performing the CSE in the sitting position. (210) The intrathecal medications consisted of 2 mL of 0.5% hyperbaric bupivacaine with 15 μg fentanyl. The left lateral position did not produce unilateral block. The left lateral position was associated with slower block onset (p = .004) but eventually produced a spinal block similar in characteristics to that obtained in the supine wedge position. The left lateral position is known to improve maternal cardiac output, and slower onset may be outweighed by the possible benefit to the fetus.
The CSE technique is a well-established method (10,58) for various types of surgery, particularly in obstetrics. In our institution, the CSE technique is the most commonly performed regional technique for labor analgesia (97%) as well as cesarean delivery (54%). CSE offers many advantages; it provides a method to administer neuraxial anesthesia and analgesia in numerous clinical situations.
The CSE technique offers the advantages of both spinal and epidural techniques and therefore has a high success rate in providing regional anesthesia. CSE provides rapid onset and the ability to titrate to a desired sensory level, control the duration of the block, and deliver postoperative analgesia. Another advantage of CSE is the facilitation of the spinal needle entrance into the subarachnoid space. The Tuohy needle serves as a guide for the spinal needle almost to the subarachnoid space. This allows for use of the smaller-gauge atraumatic spinal needles, with which the PDPH is absent or rare.(179)
The disadvantages of the CSE are that the combined technique introduces potential side effects such as PDPH, the increased risk of catheter migration into the subarachnoid space, and transient paresthesias from the spinal needle. Although the risk is low, a number of equipment modifications have been suggested and developed to avoid penetration of the epidural catheter through the dural hole made by the spinal needle.
The ideal length of spinal needle protrusion beyond the tip of the epidural needle is reported to be at least 12–13 mm. Longer spinal needles were shown to be associated with a significantly higher incidence of transient paresthesias. The inability to obtain CSF through the spinal needle may occur with shorter needles (<10 mm of protrusion) and result in failure of the spinal component of the technique. CSE failure is also related to a faulty puncture site or axis deviation during needle advancement. The risk of infection, hematoma, and neurological damage increases with multiple attempts and multiple manipulations of the needles, but it is not clear if the CSE technique increases these risks.
1. Rodgers A, Walker N, Schug S, et al. Reduction of postoperative mortality and morbidity with epidural or spinal anaesthesia: Results from overview of randomised trials. BMJ 2000;321:1493–1504.
2. Buhre W, Rossaint R: Perioperative management and monitoring in anaesthesia. Lancet 2003;362:1839–1846.
3. Kehlet H, Wilmore DW: Multimodal strategies to improve surgical outcome. Am J Surg 2002;183:630–641.
4. Holmström B, Laugaland K, Rawal N, et al: Combined spinal epidural block versus spinal and epidural block for orthopaedic surgery. Can J Anesth 1993;40:601–606.
5. Stienstra R, Dahan A, Alhadi ZRB, et al: Mechanism of action of an epidural top-up in combined spinal epidural anaesthesia. Anesth Analg 1996;83:382–386.
6. Stienstra R, Dilrosun-Alhadi BZR, Dahan A, et al: The epidural top-up in combined spinal-epidural anaesthesia: The effect of volume versus dose. Anesth Analg 1999;88:810–814.
7. Blanshard HJ, Cook TM: Use of combined spinal-epidural by obstetric anaesthetists. Anaesthesia 2004;59(9):922–923.
8. Norris MC. Are combined spinal epidural catheters reliable. Int J Obsted Anaesth 2000;9:3–6.
9. Reynolds F: Damage to the conus medullaris following spinal anaesthesia. Anaesthesia 2001;56:238–247.
10. Cook TM: Combined spinal-epidural techniques. Anaesthesia 2000; 55:42–64.
11. Hughes D, Simmons SW, Brown J, Cyna AM: Combined spinalepidural versus epidural analgesia in labour. Cochrane Database Syst Rev 2003;(4):CD003401.
12. Poulakka R, Pitkanen MT, Rosenberg PH: Comparison of technical and block characteristics of different combined spinal and epidural anesthesia techniques. Reg Anesth Pain Med 2001;26:17–23.
13. Cherng YG, Wang YP, Liu CC, Shi JJ, Huang CC: Combined spinal and epidural anesthesia for abdominal hysterectomy in a patient with myotonic dystrophy. Case report. Reg Anesth 1994;19(1):69–72.
14. Mane RS, Patil MC, Kedareshvara KS, Sanikop CS: Combined spinal epidural anesthesia for laparoscopic appendectomy in adults: A case series. Saudi J Anaesth 2012;6:27–30.
15. Browne IM, Birnbach DJ, Stein DJ, O’Gorman DA: A comparison of Espocan and Tuohy needles for the combined spinal-epidural technique for labor analgesia. Anesth Analg 2005;101:535–540.
16. Hermanides J, Hollmann MW, Stevens MF, Lirk P: Failed epidural: Causes and management. Br J Anaesth 2012;109:144–154.
17. Viscomi CM, Rathmell JP, Pace NL: Duration of intrathecal labor analgesia. Early versus advanced labor. Anesth Analg 1997;84:1108–1112.
18. Collis RE, Davies DW, Aveling W: Randomised comparison of combined spinal epidural and standard epidural analgesia in labour. Lancet 1995;345:1413–1416.
19. Palmer CM, Randall CC, Hays R, et al: The dose-response relation of intrathecal fentanyl for labor analgesia. Anesthesiology 1998;88:355–361.
20. Campbell DC, Camann WR, Datta S, et al: The addition of bupivacaine to intrathecal sufentanil for labor analgesia. Anesth Analg 1995;81: 305–309.
21. Sia AT, Chong JL, Chiu JW: Combination of intrathecal sufentanil 10 mcg plus bupivacaine 2.5 mg for labor analgesia. Is half the dose enough? Anesth Analg 1999;88:362–366.
22. Hughes D, Hill D, Fee JP: Intrathecal ropivacaine or bupivacaine with fentanyl for labour. Br J Anaesth 2001;87:733–737.
23. Vercauteren MP, Haus G, De Decker K, et al: Levobupivacaine combined with sufentanil for intrathecal labor analgesia: A comparison with racemic bupivacaine. Anesth Analg 2001;93:996–1000.
24. Van de Velde M, Dreelinck R, Dubois J, et al. Determination of the full dose-response relation of intrathecal bupivacaine, levobupivacaine, and ropivacaine, combined with sufentanil, for labor analgesia. Anesthesiology 2007;106:149–156.
25. Wilson MJ, MacArthur C, Cooper GM, Shennan A; COMET Study Group UK: Ambulation in labour and delivery mode: A randomised controlled trial of high-dose vs mobile epidural analgesia. Anaesthesia 2009;64:266–272.
26. Tsen L, Thue B, Datta S, et al: Is combined spinal-epidural analgesia associated with more rapid cervical dilation in nulliparous patients when compared with conventional epidural analgesia? Anesthesiology 1999;91: 920–925.
27. Wong CA, Scavon BM, Peaceman AM, et al: The risk of cesarean delivery with neuraxial analgesia given early versus late in labor. N Engl J Med 2005;352(7):655–665.
28. Pascual-Ramirez J, Haya J, Pérez-López FR, et al: Effect of combined spinal epidural analgesia versus epidural analgesia on labor and delivery duration. Int J Gynaecol Obstet 2011;114:246–250.
29. Carrie LES, O’Sullivan GM: Subarachnoid bupivacaine 0.5% for cesarean section. Eur J Anaesthesiol 1984;1:275–283.
30. Crowhurst J, Birnbach DJ: Low dose neuraxial block. Heading towards the new millennium. Anesth Analg 2000;90:241–242.
31. Blumgart CH, Ryall D, Dennison B, et al: Mechanism of extension of spinal anaesthesia by extradural injection of local anesthetic. Br J Anaesth 1992;169:457.
32. Kawasw H, Sumikura H, Kamada T, et al: Case of external cephalic version under combined spinal-epidural anesthesia followed by vaginal delivery. Masui 2009;58:637–640.
33. Sullivan JT, Grobman WA, Bauchat JR, et al: A randomized controlled trial of the effect of combined spinal-epidural analgesia on the success of external cephalic version for breech presentation. Int J Obstet Anesth 2009;18:328–334.
34. Pan PH, Bogard TD, Owen MD: Incidence and characteristics of failures in obstetric neuraxial analgesia and anesthesia: A retrospective analysis of 19,259 deliveries. Int J Obstet Anesth 2004;13(4):227–233.
35. Eappen S, Blinn A, Segal S: Incidence of epidural catheter replacement in parturients: A retrospective chart review. Int J Obstet Anesth 1998;7: 220–225.
36. Choi DH, Park YD: Comparison of combined spinal–epidural anaesthesia and spinal anaesthesia for caesarean section. IMRAPT 2002;14:A129.
37. Reyes M, Pan PH:. Very low-dose spinal anesthesia for cesarean section in a morbidly obese preeclamptic patient and its potential implications. Int J Obstet Anesth 2004;13:99–102.
38. Ranasinghe JS, Steadman J, Toyama T, Lai M: Combined spinal epidural anaesthesia is better than spinal or epidural alone for caesarean delivery. Br J Anaesth 2003;91:299–300.
39. Lim Y, Loo CC, Goh E: Ultra low dose combined spinal and epidural anesthesia for cesarean section. Int J Obstet Anesth 2004;13:198–200.
40. Peng PW, Chan VW, Perks A: Minimum effective anaesthetic concentration of hyperbaric lidocaine for spinal anaesthesia. Can J Anesth 1998;45:122–129.
41. Urmey WF, Stanton J, Peterson M, et al: Combined spinal–epidural anaesthesia for outpatient surgery. Dose-response characteristics of intrathecal isobaric lidocaine using a 27-gauge Whitacre needle. Anesthesiology 1995;83:528–534.
42. Norris MC, Combined spinal–epidural anaesthesia for urological and lower extremity vascular procedures. Tech Reg Anaesth Pain Manag 1997;1:131–136.
43. Patel NP, Armstrong SL, Fernando R, et al: Combined spinal epidural vs epidural labour analgesia: Does initial intrathecal analgesia reduce the subsequent minimum local analgesic concentration of epidural bupivacaine? Anesthesia 2012;67:584–593.
44. Takiguchi T, Okano T, Egawa H, et al: The effect of epidural saline injection on analgesic level during combined spinal and epidural anesthesia assessed clinically and myelographically. Anesth Analg 1997; 85:1097–1100.
45. Blumgart CH, Ryall D, Dennison B, et al: Mechanism of extension of spinal anaesthesia by extradural injection of local anaesthetic. Br J Anaesth 1992;69:457–460.
46. Loubert C, Hallworth S, Fernando R, et al: Epidural volume extension in combined spinal epidural anaesthesia for elective caesarean section: a randomised controlled trial. Anesth Analg 2011;113:811–817.
47. Doganci N, Apan A, Tekin O, Kaymak C: Epidural volume expansion: Is there a ceiling effect? Minerva Anestesiol 2010;76:334–339.
48. Tyagi A, Kumar A, Sethi AK, Mohta M: Epidural volume extension and intrathecal dose requirement: Plain versus hyperbaric bupivacaine. Anesth Analg 2008;107:333–338.
49. Tyagi A, Girotra G, Kumar A, et al: Single-shot spinal anaesthesia, combined spinal-epidural and epidural volume extension for elective caesarean section: A randomized comparison. Int J Obstet Anesth 2009; 18:231–236.
50. Fan SZ, Suseti L, Wang YP, et al: Low dose of intrathecal hyperbaric bupivacaine combined with epidural lidocaine for caesarean section—a balance block technique. Anesth Analg 1994;78:474–477.
51. Macfarlane A, Pryn A, Litchfield K, et al: Randomised controlled trial of combined spinal epidural vs. spinal anaesthesia for elective caesarean section: Vasopressor requirements and cardiovascular changes. Eur J Anaesth 2009;26:47–51.
52. Landau R, Giraud R, Morales M, Kern C, Trindade P: Sequential combined spinal-epidural anesthesia for cesarean section in a woman with a double-outlet right ventricle. Acta Anaesthesiol Scand 2004; 48: 922–926.
53. Parneix M, Fanou L, Morau E, Colson P: Low dose vombined spinalepidural anesthesia for cesarean section in a patient with Eisenmenger’s syndrome. Int J. Obstet Anesth 2009;18:81–84.
54. Agarwal A, Garg R, Joshi A, Verma S: Combined spinal epidural anesthesia with epidural volume extension technique for hysterectomy in patient with unpalliated cyanotic heart disease—A case-report. Acta Anaesthesiol Belg 2010;61:159–161.
55. Month RC, Vaida SJ: A combined spinal-epidural technique for labor analgesia and symptomatic relief in two parturients with idiopathic intracranial hypertension. Int J Obstet Anesth 2012;21:192–194.
56. Thorén T, Holmström B, Rawal N, et al: Sequential combined spinal epidural block versus spinal block for Caesarean section: Effects on maternal hypotension and neurobehavioral function of the newborn. Anesth Analg 1994;78:1087–1092.
57. Simmons SW, Cyna AM, Dennis AT, Hughes D: Combined spinalepidural versus epidural analgesia in labour. Cochrane Database Syst Rev 2007;(3):CD003401.
58. Van De Velde M: Combined spinal epidural analgesia for labor and delivery: A balanced view based on experience and literature. Acta Anaesth Belg 2009;60:109–122.
59. Hoffmann VL, Vercauteren MP, Vreugde JP, Hans GH, Coppejans HC, Adriaensen HA: Posterior epidural space depth: Safety of the loss of resistance and hanging drop techniques. Br J Anaesth 1999;83: 807–809.
60. Han KR, Kim C, Park SK, Kim JS: Distance to the adult cervical epidural space. Reg Anesth Pain Med 2003;28:95–97.
61. McAndrew CR, Harms P: Paraesthesiae during needle-through-needle combined spinal epidural versus single-shot spinal for elective caesarean section. Anaesth Intensive Care 2003;31:514–517
62. Hoffmann VL, Vercauteren MP, Buczkowski PW, Vanspringel GL: A new combined spinal-epidural apparatus: Measurement of the distance to the epidural and subarachnoid spaces. Anaesthesia 1997;52:350–355.
63. Cousins MJ, Bridenbaugh PO: Neural Blockade in Clinical Anesthesia and Management of Pain, 3rd ed. Lippincott-Raven, 1998, pp 252–255.
64. Watts RW: The influence of obesity on the relationship between body mass index and the distance to the epidural space from the skin. Anesth Intensive Care 1993;21;309–310.
65. Lirk P, Moriggl B, Colvin J, et al: The incidence of lumbar Ligamentum flavum midline gaps. Anesth Analg 2004;98:1178–1180.
66. Cook TM: Combined spinal-epidural techniques. Anaesthesia 2000;55: 42–64.
67. Landau R: Combined spinal-epidural analgesia for labor: Breakthrough or unjustified invasion? Semin Perinatol 2002;26:109–121.
68. Rawal N, Holmstrom B: The combined spinal-epidural technique. Best Pract Res Clin Anaesthesiol 2003;17:347–364.
69. Soresi AL: Episubdural anesthesia. Anesth Analg 1937;16:306–310.
70. Curelaru I: Long duration subarachnoid anesthesia with continuous epidural block. Prak Anaesth 1979;14:71–78.
71. Brownridge P: Central neural blockade and cesarean section, part 1. Review and case series. Anaesth Intensive Care 1979;7:33–41.
72. Brownridge P: Epidural and subarachnoid analgesia for elective cesarean section. Anaesthesia 1981;36:70.
73. Coates MB: Combined subarachnoid and epidural techniques. Anaesthesia. 982;37:89–90.
74. Mumtaz MH, Daz M, Kuz M: Another single space technique for orthopaedic surgery. Anaesthesia 1982;37:90.
75. Carrie LES, O’Sullivan GM: Subarachnoid bupivacaine 0.5% for cesarean section. Eur J Anaesth 1984;1:275–283.
76. Turner MA, Reifenberg NA. Combined spinal epidural analgesia. The single space double-barrel technique. Int J Obstet Anesth 1995;55: 158–160.
77. Cook TM: A new combined spinal-epidural technique. Int J Obstet Anesth 1999;55:3–6.
78. Brownridge P: Epidural and subarachnoid analgesia for elective caesarean section. Anaesthesia 1981;55:70.
79. Carrie LES: Epidural versus combined spinal epidural block for caesarean section. Acta Anaesth Scand 1988;55:5956.
80. Morris GN, Kinsella M, Thomas TA: Pencil-point needles and combined spinal epidural block. Why needle through needle? Anaesthesia 1998;55: 1132.
81. Kestin IG: Spinal anaesthesia in obstetrics. Br J Anaesth 1991;55:663.
82. Eldor J: Combined spinal–epidural anaesthesia through the Portex set. Anaesthesia 1993;55:836.
83. Soni AK, Sarna MC. Combined spinal epidural analgesia. The single space double-barrel technique. Int J Obstet Anesth 1996;55:206–207.
84. Sakuma N, Hori M, Suzuki H, et al: A sheared off and sequestered epidural catheter: A case report. Masui 2004;53(2):198–200.
85. Roberts E, Brighouse D: Combined spinal-epidural anesthesia for caesarean section. Anaesthesia 1992;55:1006.
86. Cook TM: 201 combined spinal-epidurals for anaesthesia using a separate needle technique. Eur J Anaesthesiol 2004;21:679–683.
87. Levin A, Segal S, Datta S: Does combined spinalepidural analgesia alter the incidence of paraesthesia during epidural catheter insertion? Anesth Analg 1998;55:44551.
88. Familton MJG, Morgan BM: “Needle-through-needle” technique for combined spinal–extradural anaesthesia in obstetrics. Br J Anaesth 1992; 55:327.
89. Patel M, Samsoon G, Swami A, Morgan B: Posture and the spread of hyperbaric bupivacaine in parturients using the combined spinal epidural technique. Can J Anaesth 1993;55:943–946.
90. McAndrew CR, Harms P: Paraesthesiae during needle-through-needle combined spinal epidural versus single-shot spinal for elective caesarean section. Anaesth Intensive Care 2003;31:514–517.
91. Lyons G, Macdonald R, Mikl B: Combined epiduralspinal anaesthesia for Caesarean section. Through the needle or in separate spaces? Anaesthesia 1992;55:199–201.
92. Casati A, D’ambrosio A, De Negri P, Fanelli G, Tageriello V, Tarantino F: A clinical comparison between needle-through-needle and double segment techniques for combined spinal and epidural anesthesia. Reg Anesth Pain Med 1998;55:3904.
93. Rawal N, Van Zundert A, Holmström B, Crowhurst JA: Combined spinalepidural technique. Reg Anesth 1997;55:40623.
94. Backe SK, Sheikh Z, Wilson R, Lyons GR: Combined epidural/spinal anaesthesia: Needle-through-needle or separate spaces? Eur J Anaesthesiol 2004;21:854–857.
95. Sadashivaiah J, Wilson R, McLure H, Lyons G: Double-space combined spinal-epidural technique for elective caesarean section: A review of 10 years’ experience in a UK teaching maternity unit. Int J Obstet Anesth 2010;19:183–187.
96. Van den Berg AA, Ghatge S, Wang S: Loss of resistance to saline reduces responses accompanying spinal needle insertion during institution of “needle-through-needle” combined spinal-epidural analgesia. Anaesth Intensive Care 2010;38:1013–1017.
97. Grau T, Leipold RW, Fatehi s, Martin E, Motsch J: Real-time ultrasonic observation of combined spinal-epidural anaesthesia. Eur J Anaesthesiol 2004;21:25–31.
98. Tsui BC, Gupta S, Finucane B: Determination of epidural catheter placement using nerve stimulation in obstetric patients. Reg Anesth 1999;24:17–23.
99. Herman NL, Calicott R, Van Decar TK, et al: Determination of the dose-response relationship for intrathecal sufentanil in laboring patients. Anesth Analg 1997;84:1256–1261.
100. Herman NL, Choi KC, Affleck PJ, et al: Analgesia, pruritus, and ventilation exhibit in parturients receiving intrathecal fentanyl during labor. Anesth Analg 1999;89:378–383.
101. Palmer CM, Randall CC, Hays R, et al: The dose-response relation of intrathecal fentanyl for labor analgesia. Anesthesiology 1998;88:355–361.
102. Honet JE, Arkoosh VA, Norris MC, et al: Comparison among intrathecal fentanyl, meperidine, and sufentanil for labor analgesia. Anesth Analg 1992;75:734–739.
103. Jaffe RA, Rowe MA: Comparison of the local anesthetic effects of meperidine, fentanyl, and sufentanil on dorsal root axons. Anesth Analg 1996;83:776–781.
104. Flanagan MT, Walker FO, Butterworth J: Failure of meperidine to anesthetize human median nerve. A blinded comparison with lidocaine and saline. Reg Anesth 1997;22:73–79.
105. Abouleish A, Abouleish E, Camann W: Combined spinal-epidural analgesia in advanced labor. Can J Anaesth 1994;41:575–578.
106. Campbell DC, Camann WR, Datta S: The addition of bupivacaine to intrathecal sufentanil for labor analgesia. Anesth Analg 1995;81: 305–309.
107. Sia ATH, Chong JL, Chiu JW: Combination of intrathecal sufentanil 10 μg plus bupivacaine 2.5mg for labor analgesia: Is half the dose enough? Anesth Analg 1999;88:362–366.
108. Stocks GM, Hallworth SP, Fernando R, et al: Minimum local analgesic dose of intrathecal bupivacaine in labor and the effect of intrathecal fentanyl. Anesthesiology 2001, 94:593–598.
109. Whitty R, Goldszmidt E, Parkes RK, Carvalho JC: Determination of the ED95 for intrathecal plain bupivacaine combined with fentanyl in active labor. Int J Obstet Anesth 2007;16:341–345.
110. Levin A, Datta S, Camann W: Intrathecal ropivacaine for labor analgesia: A comparison with bupivacaine. Anesth Analg 1998;87:624–627.
111. Joshi GP, McCarroll SM: Evaluation of combined spinal-epidural anaesthesia using two different techniques. Reg Anaesth 1994;55:16974.
112. Collis RE, Baxandall ML, Srikantharajah ID, Edge G, Kadim MY, Morgan BM: Combined spinal epidural analgesia with ability to walk throughout labour. Lancet 1993;55:7678.
113. Hoffmann VLH, Vercauteran MP, Buczkowski PW, Vanspringel GLJ: A new combined spinal epidural apparatus: Measurement of the distance to the epidural and subarachnoid spaces. Anaesthesia 1997;55:350–355.
114. Holloway TE, Telford RJ: Observations on deliberate dural puncture with a Touhy needle: Depth measurement. Anesthesia 1991;46:722–724.
115. Cousins MJ, Bridenbaugh PO: Neural Blockade in Clinical Anesthesia and Management of Pain, 3rd ed. Lippincott-Raven, 1998, pp 255.
116. Brighouse D, Wilkins A: Failure of pencil-point spinal needles to enter the subarachnoid space. Anaesthesia 1994;55:176.
117. Meiklejohn BH: The effect of rotation of an epidural needle: An in vitro study. Anaesthesia 1987;42:1180–1182.
118. Husemeyer RP, White DC: Topography of the lumbar epidural space. Anaesthesia 1980;55:7–11.
119. Waldman SA, Liguori GA: Comparison of the flow rates of 27-Gauge Whitacre and Sprotte needles for combined spinal and epidural anesthesia. Reg Anesth 1996;55:378–379.
120. Vandermeersch E: Combined spinal-epidural anaesthesia. Balliere’s Clin Anaesth 1993;7:691–708.
121. Fukishige T, Sano T, Kano T: Lumbar dural sac deformation after epidural injection. Anesthesiology 1998;55:A870.
122. Norris MC, Grieco WM, Borkowski M, et al: Complications of labor analgesia: Epidural versus combined spinal epidural techniques. Anesth Analg 1994;55:529–537.
123. Lesser P, Bembridge M, Lyons G, Macdonald R: An evaluation of a 30-gauge needle for spinal anaesthesia for Caesarean section. Anaesthesia 1990;55:76–78.
124. Dennison B: Combined subarachnoid and epidural block for caesarean section. Can J Anaesth 1987;55:105.
125. Patel M, Swami M: Combined spinal-extradural anaesthesia for Caesarean section. Anaesthesia 1992;55:1005–1006.
126. Robbins PM, Fernando R, Lim GH: Accidental intrathecal insertion of an extradural catheter during combined spinal-extradural anaesthesia for Caesarean section. Br J Anaesth 1995;75:355–357.
127. Vucevic M, Russell IF: Spinal anaesthesia for caesarean section: 0.125% plain bupivacaine 12mL compared with 0.5% plain bupivacaine 3 ml. Br J Anaesth 1992;55:590–595.
128. Ferguson DJM: Dural puncture and epidural catheters. Anaesthesia 1992;55:272.
129. Muranaka K, Tsutsui T: Comparison of clinical usefulness of the two types of combined spinal epidural needles. Masui 1994;55:1714–1717.
130. Angle P, Kronberg JE, Thompson DE, Duffin J, et al: Epidural catheter penetration of human dura tissue: In vitro investigation. Anesthesiology 2004;100(6);141–146.
131. Holtz D, Mollman M, Schymroszcyk B, Ebel C, Wendt M: No risk of metal toxicity in combined spinal-epidural anesthesia. Anesth Analg 1999;88(2):393–397.
132. Holmstrom B, Rawal N, Axelsson K, et al: Risk of catheter migration during combined spinal epidural block-percutaneous epiduroscopy study. Anesth Analg 1995;80:747–753.
133. Leighton BL, Arkoosh VA, Huffnagle S, et al: The dermatomal spread of epidural bupivacaine with and without prior intrathecal sufentanil. Anesth Analg 1996;83:526–529.
134. Stienstra R, Dilrosun-Alhadi BZ, Dahan A, van Kleef JW, Veering BT, Burm AG: The epidural “top-up” in combined spinal-epidural anesthesia: The effect of volume versus dose. Anesth Analg 1999;88:810–814.
135. Suzuki N, Koganemaru M, Onizuka S, Takasaki M: Dural puncture with a 26G spinal needle affects spread of epidural anesthesia. Anesth Analg 1996;82:1040–1042.
136. Kamiya Y, Kikuchi T, Inagawa G, et al: Lidocaine concentration in cerebrospinal fluid after epidural administration: A comparison between epidural and combined spinal-epidural anesthesia. Anesthesiology 2009;110:1127–1132.
137. Leach A, Smith GB: Subarachnoid spread of epidural local anaesthetic following dural puncture. Anaesthesia 1988;43:671–674.
138. Gaiser RR, Lewin SB, Cheek TG, Gutsche BB: Effects of immediately initiating an epidural infusion in the combined spinal and epidural technique in nulliparous parturients. Reg Anesth Pain Med 2000;25: 223–227.
139. Beaubien G, Drolet P, Girard M, Grenier Y: Patient-controlled epidural analgesia with fentanyl-bupivacaine: Influence of prior dural puncture. Reg Anesth Pain Med 2000;25:254–258.
140. Hodgkinson R: Total spinal block after epidural injection into an interspace adjacent to an inadvertent dural perforation. Anesthesiology 1981;55:593–595.
141. Eldor J, Guedj P, Levine S. Delayed respiratory arrest in combined spinal-epidural anesthesia. Case report. Reg Anesth 1994;19; 418–422.
142. Kuczkowski KM, Birnbach DJ, O’Gorman DA, Stein DJ, Santos AC: Does a test dose increase the likelihood of identifying intrathecal placement of epidural catheters during labor analgesia? Abstract of Scientific Papers SOAP. Anesthesiology 2000;A26.
143. Goy RW, Sia AT. Sensorimotor anesthesia and hypotension after subarachnoid block: Combined spinal-epidural versus single-shot spinal technique. Anesth Analg 2004;98(2):491–496.
144. Goy RWL, Chee-Seng Y: The median effective dose of intrathecal hyperbaric bupivacaine is larger in the single-shot spinal as compared with the combined spinal-epidural technique. Anesth Analg 2005; 100: 1499–1502.
145. Gaur V, Gupta RK, Agarwal A, et al: Air or nitrous oxide for loss-ofresistance epidural technique? Can J Anaesth 2000;47:503–505.
146. Carpenter RL, Hogan QH, Liu SS, et al: Lumbosacral cerebrospinal fluid volume is the primary determinant of sensory block extent and duration during spinal anesthesia. Anesthesiology 1998;89:24–29.
147. Rawal N, Schollin J, Wesström G: Epidural versus combined spinal epidural block for caesarean section. Acta Anaesth Scand 1988;32: 61–66.
148. Kumar C: Combined subarachnoid and epidural block for caesarean section. Can J Anesth 1987;34:329–330.
149. Mandell GL, Jamnback L, Ramanathan S: Hemodynamic effects of subarachnoid fentanyl in laboring parturients. Reg Anesth 1995;21(2): 103–111.
150. Horlocker TT, McGregor DG, Matsushige DK, Schroeder DR, Besse JA: A retrospective review of 4767 consecutive spinal anesthetics: Central nervous system complications. Anesth Analg 1997;55:578–584.
151. Bigeleisen PE: Nerve puncture and apparent intraneural injection during ultrasound-guided axillary block does not invariably result in neurologic injury. Anesthesiology 2006;105:779–783.
152. Simsa J: Use of 29-G spinal needle s and a fixation device with combined spinal epidural technique. Acta Anaesth Scand 1994;38:439–441.
153. McAndrew CR, Harms P: Paraesthesiae during needle-through-needle combined spinal epidural versus single-shot spinal for elective caesarean section. Anaesth Intensive Care 2003;31(5):514–517.
154. Holloway J, Seed PT, O’Sullivan G, Reynolds F: Paraesthesiae and nerve damage following combined spinal epidural and spinal anaesthesia: A pilot survey. Int J Obstet Anesth 2000;9(3):151–155.
155. Turner MA, Shaw M: Atraumatic spinal needlea [letter]. Anaesthesia 1993;48:452.
156. Broadbent CR, Maxwell WB, Ferrie R, et al: Ability of anaesthetists to identify a marked lumbar interspace. Anaesthesia 2000;55:1106–1126.
157. Eldor J: Metallic fragments and the combined spinal-extradural technique. Br J Anaesth 1992;69:663.
158. Sharma B, Gupta S, Jain N, Handoo A, Sood J: Cerebrospinal fluid cytology in patients undergoing combined spinal epidural versus spinal anaesthesia without an introducer. Anaesth Intensive Care 2011; 39: 914–918.
159. Dripps Rd, Vandem LD: Long-term follow-up of patients who received 10,098 spinal anaesthetics. JAMA 1954;156:1486–1491.
160. Phillips OC, Ebner H, Melson AT, Black MH: Neurological complications following spinal anesthesia with lidocaine: A prospective review of 10,440 cases. Anesthesiology 1969;30:284–289.
161. Harding SA, Collis RE, Morgan BM: Meningitis after combined spinal extradural anaesthesia in obstetrics. Br J Anaesth 1994;73:545–547.
162. Cascio M: Meningitis following a combined spinal-epidural technique in a labouring term parturient. Can J Anaesth 1996;43:399–402.
163. Pinder AJ, Dresner M: Meningococcal meningitis after combined spinalepidural analgesia. Int J Obstet Anesth 2003;12:183–187.
164. McLure HA, Talboys CA, Yentis SM, Azadian BS: Surgical facemesks and downward dispersal of bacteria. Anaesthesia 1998;53:624–626.
165. Phillips BJ, Fergusson S, Armstrong P, Anderson FM, Wildsmith JAW: Surgical facemasks are effective in reducing bacterial contamination caused by dispersal from the upper airway. Br J Anaesth 1992;69:407–408.
166. Burnstein R, Buckland R, Pickett JA: A survey of epidural analgesia for labour in the United Kingdom. Anesthesia 1999;54:634–650.
167. Siegel J, Rhinehart E, Jackson M, Chiarello L; and the Healthcare Infection Control Practices Advisory Committee. Guideline for isolation precautions: Preventing transmission of infectious agents in healthcare settings, June 2007. http://www.premierinc.com/safety/topics/ guidelines/downloads/cdc-isolation-2007.pdf.
168. Centers for Disease Control and Prevention. Bacterial meningitis after spinal anesthesia during labor—Ohio and New York, 2008–2009. MMWR Morb Mortal Wkly Rep 2010;59:65–69.
169. Sandkovsky U, Mihu MR, Adeyeye A, et al: Iatrogenic meningitis in an obstetric patient after combined spinal-epidural analgesia: Case report and review of the literature. South Med J 2009;102:287–290.
170. Choy JC: Mortality from peripartum meningitis. Anaesth Intensive Care 2000;28:328–330.
171. Tariq A: Neurological deficit following combined spinal-epidural anesthesia for knee arthroplasty. Middle East J Anesthesiol 2010;20:759–762.
172. Takasu, M, Okita M, Araki m, et al: Gadolinium enhancement of cauda equina after combined spinal-epidural anaesthesia. Br J Radiol 2010;83: 192–194.
173. Kubina P, Gupta A Oscarsson A, et al: Two cases of cauda equina syndrome following spinal-epidural anesthesia. Reg Anesth 1997;22: 447–450.
174. Kato J, Konishi J, Yoshida H, et al: Cauda equina syndrome following combined spinal and epidural anesthesia: A case report. Can J Anaesth 2011;58:638–641.
175. Rawal N, Holmstrom B, Croehurst JA, Van Zundert A: The combined spinal-epidural technique. Anaesthsiol Clin North America 2000;18: 267–295.
176. Dunn SM, Connelly NR, Parker RK: Postdural puncture headache (PDPH) and combined spinal anesthesia (CSE). Anesth Analg 2000;90: 1249–1250.
177. Balestrieri PJ: The incidence of postdural puncture headache and combined spinal-epidural: Some thoughts. Int J Obstet Anesth 2003; 12(4):305–306.
178. Brownridge P. Spinal anaesthesia in obstetrics. Br J Anaesth 1991;67: 663–667.
179. Geurts JW, Haanschoten MC, Van Wijk RM, Kraak H, Besse TC: Postdural puncture headache in young patients. A comparative study between the use of 0.52 mm (25-gauge) and 0.33 mm (29-gauge) spinal needles. Acta Anaesthesiol Scand 1990;34:350–353.
180. Chan BO, Paech MJ: Persistent cerebrospinal fluid leak: A complication of the combined spinal-epidural technique. Anesth Analg 2004;98(3): 828–830.
181. Reisinger PWM, Hochstrasser K: The diagnosis of CSF fistulae on the basis of detection of beta2-transferrin by polyacrylamide gel electrophoresis and immunoblotting. J Clin Chem Clin Biochem 1989; 27:169–172.
182. Howes J, Lenz R: Cerebrospinal fluid cutaneous fistula—An unusual complication of epidural anaesthesia. Anaesthesia 1994;49:221–222.
183. Pan PH, Moore CH, Ross VH: Severe maternal bradycardia and asystole after combined spinal-epidural labor analgesia in a morbidly obese parturient. J Clin Anesth 2004;16(6):461–464.
184. Kuczkowski KM: Severe persistent fetal bradycardia following subarachnoid administration of fentanyl and bupivacaine for induction of a combined spinal-epidural analgesia for labor pain. J Clin Anesth 2004;16(1):78–79.
185. D’Angelo R, Eisenach JC: Severe maternal hypotension and fetal bradycardia after a CSE. Anesthesiology 1997;81:116–118.
186. Mardirosoff C, Dumont L, Boulvain M, Tramèr MR: Fetal bradycardia due to intrathecal opioids for labour analgesia: A systematic review. BJOG 2002;109:274–281.
187. Van de Velde M: Neuraxial analgesia and fetal bradycardia. Curr Opin Anaesthesiol 2005;18:253–256.
188. Nocolet J, Miller A, Kaufman I, et al: Maternal factors implicated in fetal bradycardia after combined spinal epidural for labour pain. Eur J Anaesth 2008;25:721–725.
189. Clarke VT, Smiley RM, Finster M: Uterine hyperactivity after intrathecal injection of fentanyl for analgesia durign labor: A cause of fetal brady cardia? Anesthesiology 1994;81:1083.
190. Albright GA, Forster RM: Does combined spinal-epidural analgesia with subarachnoid sufentanil increase the incidence of emergency cesarean delivery? Reg Anesth Pain Med 1997;22:400–405.
191. Skupski DW, Abramovitz S, Samuels J, et al: Adverse effects of combined spinal-epidural versus traditional epidural analgesia during labor. Int J. Gynecol Obstet 2009;106:242–245.
192. Rawal N, Schollin J, Wesstrom G: Epidural versus combined spinal epidural block for cesarean section. Acta Anaesthesiol Scand 1988;32: 61–66.
193. Joshi GP, MaCarroll SM: Combined spinal–epidural anesthesia using needle-through-needle technique [Letter]. Anesthesiology 1993;78: 406–407.
194. Pan PH: Laboratory evaluation of single-lumen, dual-orifice combined spinal-epidural needles: Effects of bevel orientation and modified technique. J Clin Anesth 1998;10(4):286–290.
195. Riley ET, Hamilton CL, Ratner EF, Cohen S: A comparison of the 24-gauge Sprotte and Gertie Marx spinal needles for combined spinalepidural analgesia during labor. Anesthesiology 2002;97:574–577.
196. Herbstman CH, Jaffe JB, Tuman KJ, et al: An in vivo evaluation of four spinal needles used for the combined spinal-epidural technique. Anesth Analg 1998;86(3):520–522.
197. Simsa J: Needle fixation with combined spinalepidural anaesthesia. Acta Anaesth Scand 1995;55:275.
198. Stocks GM, Hallworth SP, Fernando R: Evaluation of a spinal needle locking device for use with the combined spinal epidural technique. Anaesthesia 2000;55(12):1185–1188.
199. Eldor J, Chaimsky G: The Eldor combined-spinal epidural needle. Anesthesia 1993;48:173.
200. Torrieri A, Aldrete JA: Combined spinal-epidural needle. Acts Anaesth Belg 1998;39:65–66.
201. Birnbach DJ, Chestnut DH: The epidural test dose in obstetric practice: Has it outlived its usefulness? Anesth Analg 1999;88:971.
202. Steffek M, Owczuk R, Szlyk-Augustyn M, Lasinska-Kowara M, Wujtewicz M: Total spinal anaesthesia as a complication of local anaesthetic test-dose administration through an epidural catheter. Acta Anaesthesiol Scand 2004;48(9):1211–1213.
203. Norris MC, Ferrenbach D, Dalman H, et al: Does epinephrine improve the diagnostic accuracy of aspiration during labor epidural analgesia? Anesth Analg 1999;88;1073.
204. Moore DC, Batra MS: The components of an effective test dose prior to epidural block. Anesthesiology 1981;55:693.
205. Hood DD, Dewan DM, James FM III: Maternal and fetal effect of epinephrine in gravid ewes. Anesthesiology 1986;64:610.
206. Leighton BL, Norris MC, DeSimone CA, et al: The air test as a clinically useful indicator of intravenously placed epidural catheters. Anesthesiology 1990;73:610.
207. Kopacz DJ: Spinal 2-chloroprocaine: Minimum effective dose. Reg Anesth Pain Med 2005;30(1):36–42.
208. Hamza J, Smida M, Benhamou D, Cohen SE: Parturient’s poture during epidural puncture affects the distance from skin to epidural space. J Clin Anesth 1995;7:1–4.
209. Yun EM, Marx GF, Santos AC: The effects of maternal position during induction of combined spinal-epidural anesthesia for caesarean delivery. Anesth Analg 1998;87:614–618.
210. Lewis NL, Ritchie EL, Downer JP, Nel MR: Left lateral vs. supine, wedged position for development of block after combined spinal-epidural anaesthesia for caesarean section. Anaesthesia 2004;59: 894–898.
Based on J. Sudharma Ranasinghe, Elyad Davidson, and David J. Birnbach from Hadzic’s textbook of Regional Anesthesia and Acute Pain Management” second edition.