
The foot plays an essential role in standing and locomotion. It supports the body weight in standing, levers it forward in walking, and absorbs shock in running and jumping. The foot can be affected by a variety of congenital, inflammatory, infectious, degenerative, and neoplastic disorders. Based on its dynamic and resolution capabilities, US is an efficient means of detecting and assessing foot disorders. (Rockett 1999; Rawool and Nazarian 2000; D’Agostino et al. 2005; Borman et al. 2005; Sabir et al. 2005). Parts of this chapter (i.e., tendons, nerves) are linked with what has already been described in the chapter on the ankle.
The foot is characterized by a complex anatomy: it is formed by 28 bones, 30 joints, and more than 100 muscles, tendons, and ligaments. Specific anatomic references are conventionally used when examining the foot, movements in the transverse plane being referred to the midline of the foot, which is defined as the long axis of the second toe, and not to the midline of the body. As a consequence, adduction means movement toward the second toe and abduction means motion away from it. The abductor hallucis muscle, which lies on the medial edge of the plantar foot, is referred to as an abductor muscle because it moves the hallucis away from the midline of the foot, but it should be regarded as an adductor if the midline of the body is kept as the reference.
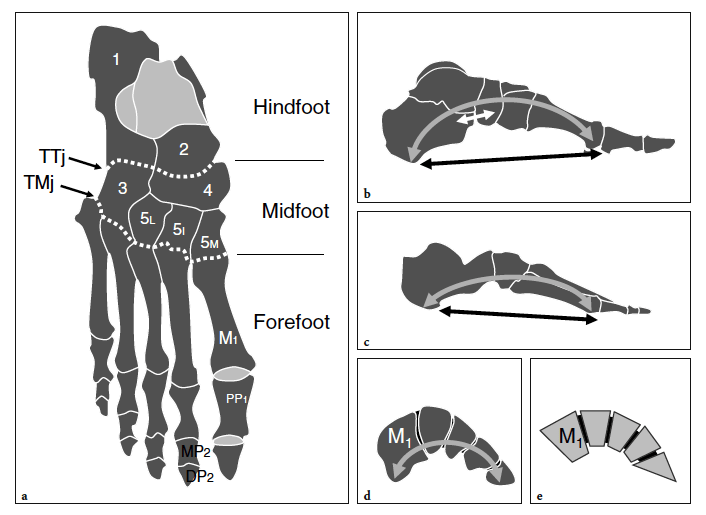
In terms of topographic bone anatomy, the foot can be subdivided into three parts: the hindfoot (talus and calcaneus), the midfoot (navicular, cuboid, and the three cuneiforms), and the forefoot (metatarsals and phalanges) (Fig. 1a). Each of these parts consists of several joints. The subtalar (talocalcaneal) joint is formed by the large concave facet located on the inferior aspect of the talus and the convex posterior articular surface of the superior aspect of the calcaneus. The transverse tarsal joint, consisting of the talonavicular joint medially and the calcaneocuboid joint laterally, allows inversion (inward rotation) and eversion (outward rotation) movements of the foot. In a more distal location, the navicular bone articulates with the three cuneiforms: the first, the medial; the second, the middle; and the third, the lateral. Then, the cuneiforms and the cuboid articulate with the base of the five metatarsals forming the tarsometatarsal joint. More distally, the forefoot joints, i.e., metatarsophalangeal, proximal and distal interphalangeal joints, allow graded flexion and extension of the great and the lesser toes.
The bones of the foot do not lie on a flat plane. They are arranged to build three main arches, each characterized by inferior concavity: the medial longitudinal, the lateral longitudinal, and the transverse. The medial longitudinal arch is formed by the calcaneus, the talus, the navicular, the three cuneiforms, and the first three metatarsals (Fig.1b). This arch is concave inferiorly and is stabilized by the combined action of ligaments and muscles. The main ligament stabilizing the medial longitudinal arch is the plantar aponeurosis, which joins the two pillars of the arch: the posteroinferior aspect of the calcaneus and the three medial proximal phalanges. The plantar calcaneonavicular ligament (spring ligament) joins the navicular and the calcaneus and supports the talar head, thus contributing to the maintenance of the arch. Some muscles have a stabilizing role. Because of its median position, the flexor hallucis longus acts as a bowstring of the arch. In addition, the tibialis posterior and the anterior muscles of the leg contribute to maintaining the concavity of the arch by inverting and adducting the foot, so helping to raise its medial border. Other intrinsic muscles play a role but to a lesser extent. The lateral longitudinal arch is formed by the calcaneus, the cuboid, and the fourth and fifth metatarsals (Fig. 1c). The pillars are the calcaneus and the lateral two metatarsal heads. Similar to the medial longitudinal arch, the inferior concavity of the lateral arch is, for the most part, maintained by ligament structures and the lateral extension of the plantar aponeurosis. The peroneus longus tendon plays an important role as a bowstring of this arch. The bones involved in the anterior transverse arch are the bases of the five metatarsals, the cuboid, and the cuneiforms (Fig.1d,e). This anterior transverse arch results from the shape of the distal row of tarsal bones (wedge-shaped intermediate and lateral cuneiforms). The stability of the anterior transverse arch is assured by several ligaments and the peroneus longus tendon. At the level of the metatarsal heads, the anterior transverse arch is less concave and maintained by the action of the deep transverse ligament that connects the plantar aspect of the metatarsal heads together.
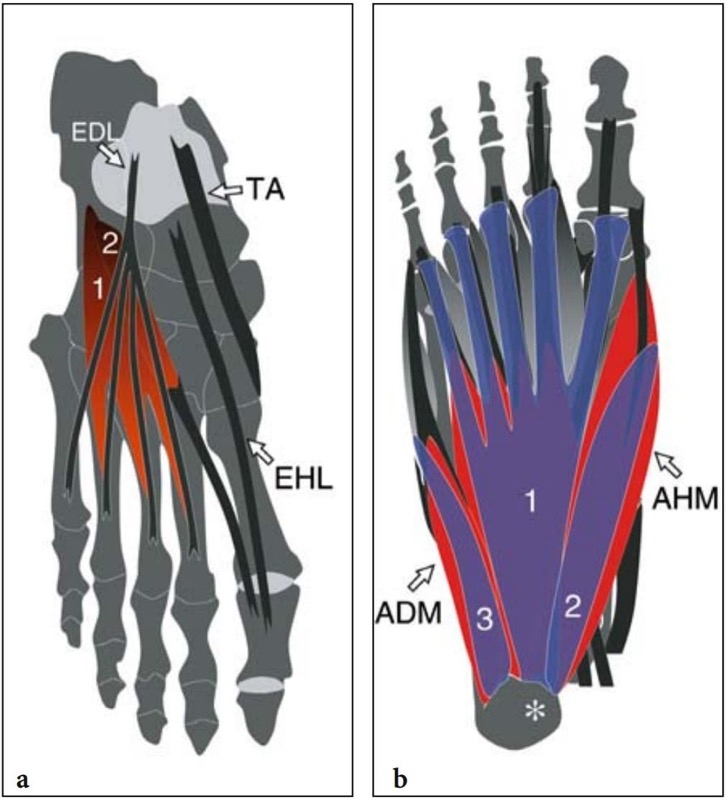
The skin overlying the dorsum of the foot is thin, with a mean epidermal thickness of 0.064 mm and sparse subcutaneous fat. The sensory supply is guaranteed by distal branches of the saphenous nerve (medial side), the superficial peroneal nerve (central and lateral side), and the sural nerve (lateral border of the foot), while the terminal branches of the deep peroneal nerve supply the skin over the dorsum of the first web space. Deep to the thin deep fascia, the tendons of the tibialis anterior, extensor hallucis longus, and extensor digitorum longus travel down to insert distally into the anteromedial aspect of the first cuneiform and the base of the first metatarsal, the distal phalanx of the hallux, and the distal phalanges of the lesser toes, respectively (Fig. 2a). The intrinsic muscles of the dorsum of the foot are the short extensors of the toes: the extensor digitorum brevis and the extensor hallucis brevis. The extensor digitorum brevis takes its origin from the anterolateral part of the superior aspect of the calcaneus and inserts onto the lateral sides of the tendons of the extensor digitorum longus for the second, third, and fourth toes. The extensor hallucis brevis muscle represents the medial part of the extensor digitorum brevis and may be more or less distinct from it, continues distally into a thin tendon that runs laterally to the extensor hallucis longus, and inserts into the dorsal aspect of the proximal phalanx of the great toe. Both muscles are innervated by the deep peroneal nerve.
The dorsalis pedis artery is the direct continuation of the anterior tibial artery and represents the main vascular supply for the toes: it begins midway between the lateral and medial malleolus and runs anteromedially between the tendons of the extensor hallucis longus and extensor digitorum longus to reach the first interosseous space. At the level of the tarsometatarsal joint, the dorsalis pedis artery gives off the first metatarsal artery and an arcuate artery which sends branches to the second, third, and fourth metatarsals. Lateral to the dorsalis pedis artery, the medial branch of the deep peroneal nerve is directed straight forward to reach the first intermetatarsal space.
In contrast to the dorsum of the foot, the skin covering the sole is significantly thicker – the epidermis is approximately 8 times thicker than that of the dorsum – and richly innervated. The plantar sensory supply depends on the medial plantar nerve (medial side) and the lateral plantar nerve (lateral side). Calcaneal branches of the tibial and sural nerve gives off sensory branches for the skin of the heel. The skin lies on a subcutaneous tissue layer that is very thick posteriorly (heel pad) in order to resist the impaction forces and attrition exerted during walking or running. The special anatomic arrangement of the heel fat pad allows these forces to be withstood: it has an average thickness of 18 mm and is formed by multiple fat-containing cells separated by vertical fibrous and elastic septa that arise from the deep aspect of the skin and insert into the superficial aspect of the plantar fascia, or plantar aponeurosis. This peculiar arrangement allows the subcutaneous tissue to act as a shockabsorber during walking and running, thus limiting the damage induced by compressive loads. At the midfoot level, the subcutaneous tissue becomes progressively thin and then thickens again under the metatarsophalangeal joints in order to lessen loads to the deep structures during the toe-off phase of gait. Deep to the subcutaneous tissue, the plantar fascia is a fibrous thickening of the superficial fascia which acts as a dynamic support for the longitudinal arches of the foot. The plantar fascia is made up of a network of compacted collagen fibers, most of which are oriented longitudinally and, to a lesser extent, transversely. Interspersed elastic tissue allows some plastic elongation of the plantar fascia during weight-bearing. The plantar fascia consists of three cords: a central cord, which is the thickest, the largest and the strongest, and two thinner medial and lateral cord. The central cord has a triangular shape, is thicker posteriorly, and fans out becoming progressively thinner anteriorly. It arises from the medial tubercle of the calcaneal tuberosity and divides into five diverging bands at the mid-metatarsal level. At the metatarsophalangeal joints, each band splits to enclose the flexor tendons of the toes and then inserts into the fibrous digital sheath and the base of the respective proximal phalanx. The thin medial band covers the abductor hallucis muscle and blends distally with its fascia; on the other side, the lateral band overlies the abductor digiti minimi and inserts into the base of the fifth metatarsal. The lateral cord may be absent. The plantar fascia maintains the medial and the lateral longitudinal arch, gives a firm attachment to the overlying skin, and protects the underlying vessels, nerves, and tendons from direct trauma.
The plantar muscles of the foot are conventionally grouped in four layers. The first is the most superficial and houses the abductor hallucis, the flexor digitorum brevis, and the abductor digiti minimi muscles (Fig. 3a). The abductor hallucis is a large muscle on the medial side of the sole that takes its origin from the medial tubercle and the medial surface of calcaneus and the medial border of plantar aponeurosis. It attaches to the medial aspect of the base of the proximal phalanx of the great toe. The abductor hallucis acts as an abductor (from the anatomic axis of the foot) and plays a secondary role as a flexor of the great toe at the first metatarsophalangeal joint. In patients with hallux valgus, this muscle is pulled plantarward and becomes unable to abduct the great toe. The flexor digitorum brevis arises from the deep surface of the plantar fascia and the medial tubercle of the calcaneal tuberosity and gives rise to four tendons that reach the four lesser toes. More distally, each tendon enters the flexor fibrous sheath and splits into two slips – similarly to the flexor digitorum superficialis of the hand – to pass on each side of the flexor digitorum longus and insert into the sides of the shaft of the middle phalanx of the corresponding toe. The muscle flexes the lateral four toes at the proximal interphalangeal joints. The abductor digiti minimi is a slender muscle arising from the medial and lateral tubercles of calcaneus and the lateral border of the plantar fascia and inserting onto the base of the proximal phalanx of the fifth toe. It acts as an abductor of the fifth toe at the metatarsophalangeal joint. The abductor hallucis and flexor digitorum brevis muscles are supplied by the median plantar nerve, and the abductor digiti minimi by the lateral plantar nerve.
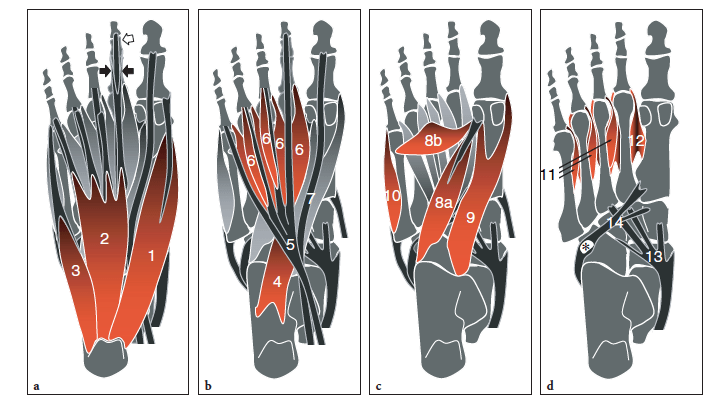
The second layer contains the quadratus plantae and the lumbricals together with the tendons of the flexor hallucis longus and flexor digitorum longus (Fig. 3b). The quadratus plantae, which is also referred to as the flexor digitorum accessorius, has an extensive origin from the medial surface and the medial tubercle of the calcaneus (medial head) and the inferior surface and the lateral tubercle of the calcaneus (lateral head). The two heads insert into the lateral aspect of the flexor digitorum longus tendon at the point where the latter gives off its four tendons. The quadratus plantae assists the action of the flexor digitorum longus by straightening the line of pull of this tendon. The four lumbricals are slender muscles arising from the respective tendinous slips of the flexor digitorum longus, immediately distal to the point where they begin to diverge. Distally, the lumbricals insert into the medial aspect of the dorsal extensor hood of the second to the fifth toes. Both quadratus plantae and lumbricals are innervated by the lateral plantar nerve.
The third layer of intrinsic foot muscles is occupied by the flexor hallucis brevis, the adductor hallucis, and the flexor digiti minimi brevis muscles (Fig. 3c). The flexor hallucis brevis takes its origin from the plantar surface of the cuboid, posterior to the peroneal groove, and the lateral cuneiform (lateral head) and the plantar surfaces of the medial and intermediate cuneiforms, blending in this region with expansions of the tibialis posterior tendon (medial head). The muscle belly derived from the union of the two heads has two distal tendons which insert into the medial and lateral sides of the base of the proximal phalanx of the great toe, blending with the insertions of the abductor hallucis (medial head) and adductor hallucis (lateral head). Each tendon contains a sesamoid (medial and lateral) which lies under the head of the first metatarsal. The flexor hallucis brevis is a flexor of the first metatarsophalangeal joint. The adductor hallucis consists of a larger transverse head and a smaller oblique head, both converging distally to form a single short tendon. The transverse head arises from the surface of the plantar ligaments of the third, fourth, and fifth metatarsophalangeal joints and from the deep transverse metatarsal ligament which bridges the metatarsal heads; the oblique head arises from the bases of the second, third, and fourth metatarsals. Distally, the adductor hallucis attaches to the lateral aspect of the base of the proximal phalanx of the great toe, blending with the tendon of the flexor hallucis brevis and sending fibers to the lateral sesamoid. Both heads of the adductor hallucis are adductors of the great toe, while the oblique plays an additional role in flexion and in maintaining the transverse arch. The flexor digiti minimi brevis arises from the plantar surface of the base of fifth metatarsal and the sheath of the peroneus longus tendon and attaches to the base of the proximal phalanx of the fifth toe. It is a flexor of the fifth toe at the metatarsophalangeal joint. The flexor hallucis brevis is innervated by the medial plantar nerve; the adductor hallucis and the flexor digiti minimi brevis are innervated by the lateral plantar nerves.
Finally, the interosseous muscles (four dorsal and three plantar) lie in the fourth and deepest layer, within the intermetatarsal spaces (Fig. 3d). The dorsal interosseous muscles arise from the adjacent facing surfaces of the metatarsal shafts and insert into the lateral surface of the base of the proximal phalanx of the toes (except for the first one which inserts onto the medial surface of the second toe). The plantar interosseous muscles are found immediately plantar to the dorsal ones. They arise from the plantar and medial aspects of the base of the third, fourth, and fifth metatarsals and attach into the medial surface of the base of the proximal phalanx of the respective toes. Both dorsal and ventral interosseous muscles receive branches from the lateral plantar nerve.
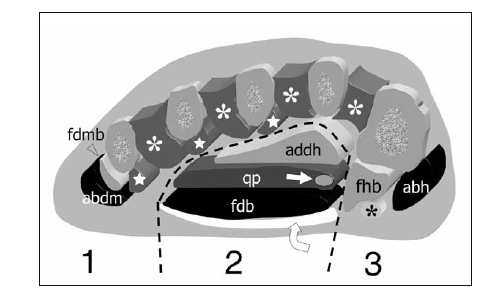
Several tendons of extrinsic muscles of the foot, such as the tibialis posterior, the flexor hallucis longus and the flexor digitorum longus, the peroneus brevis and longus, travel in close proximity to the plantar aspect of the tarsal bones. After crossing the medial malleolus, the tibialis posterior assumes a straight course to fan out and insert into the tuberosity of the navicular, sending extensions to the cuneiforms and the bases of the second to fourth metatarsals: it acts as an invertor and plantar flexor of the foot (Fig. 3d). The distal tibialis posterior tendon may house an accessory ossicle within, the so-called os tibiale externum (accessory navicular). The flexor hallucis longus passes on the undersurface of the sustentaculum tali and, in the sole of the foot, crosses the tendon of the flexor digitorum longus, to which it is connected by a fibrous slip; then, it passes between the medial and lateral sesamoids at the head of the first metatarsal to attach into the base of the distal phalanx of the great toe (Fig. 3b). The flexor hallucis longus tendon flexes the great toe and assists in plantar flexion of the foot at the ankle joint as well as in the maintenance of the medial longitudinal arch. The flexor digitorum longus passes superficial to the sustentaculum tali and runs obliquely into the sole of the foot crossing deep to the abductor hallucis and the flexor digitorum brevis muscles and plantar to the flexor hallucis longus tendon (Fig. 3b). More distally, it divides into four slips which insert into the plantar surface of the distal phalanges of the second to the fifth toes. The flexor digitorum longus flexes the phalanges of the lesser toes and also acts as a plantar flexor of the ankle joint. On the lateral sole, the peroneus brevis has a straight course to insert into the styloid process of the base of the fifth metatarsal, whereas the peroneus longus courses obliquely forward in a plantar direction toward the cuboid (Fig. 3d). At that point, the peroneus longus enters an osteofibrous tunnel beneath the cuboid and traverses the sole of the foot, from lateral to medial, to insert into the base of the first metatarsal and the lateral surface of the medial cuneiform. In 25% of cases, the peroneus longus tendon may contain a sesamoid bone, the “os peroneum”, at the point where it turns around the lateral border of the foot. The peroneal tendons are evertors of the foot and play a secondary role as plantar flexors of the ankle joint; the peroneus longus also supports the lateral longitudinal and transverse arches of the foot. Figure 4 illustrates the four layers of intrinsic muscles on a cross-sectional view of the foot. Arising from the deep aspect of the plantar fascia, two vertical fibrous septa, medial and lateral, divide the plantar aspect of the foot into three compartments: medial, central, and lateral. The medial compartment contains the abductor hallucis muscle, the flexor hallucis brevis muscle, and the flexor hallucis longus tendon; the central compartment houses three layers of muscles, including the flexor digitorum brevis, quadratus plantae, lumbricals, adductor hallucis, and the flexor digitorum longus tendon; the lateral compartment houses the flexor and abductor digiti minimi brevis muscles. Awareness of these compartments is essential when evaluating the spread of soft-tissue infections and tumors.
The main arteries of the sole of the foot are the large lateral and the small medial plantar artery that take their origin from the posterior tibial artery. Both pass forward deep to the abductor hallucis to reach the sole together with the two terminal branches of the tibial nerve, the medial and lateral plantar nerves. The lateral plantar artery ends at the base of the first metatarsal bone, where it joins the deep plantar branch of the dorsalis pedis artery to form the plantar arterial arch.
The medial and lateral plantar nerves supply the skin and the intrinsic muscles (except for the extensor digitorum brevis, which is innervated by the deep peroneal nerve) of the foot. The medial plantar nerve is the larger and courses deep to the abductor hallucis muscle and then between this muscle and the flexor hallucis brevis, alongside the medial plantar artery. At the level of the metatarsal bases, the medial plantar nerve divides into three digital nerves that travel in the web spaces. The smaller lateral plantar nerve runs deep to the abductor hallucis and then courses anterolaterally, between the first and second layers of plantar muscles. It divides into a superficial and a deep branch: the superficial splits into two digital nerves. Among the digital nerves, the third one is thicker because it derives from the fusion of two nerve branches which arise from the medial and lateral plantar nerves. The anatomy of the intermetatarsal spaces will be discussed in more detail later. The sensory supply of the lateral margin of the foot is provided by the sural nerve, whereas the innervation of its medial margin belongs to the saphenous nerve.
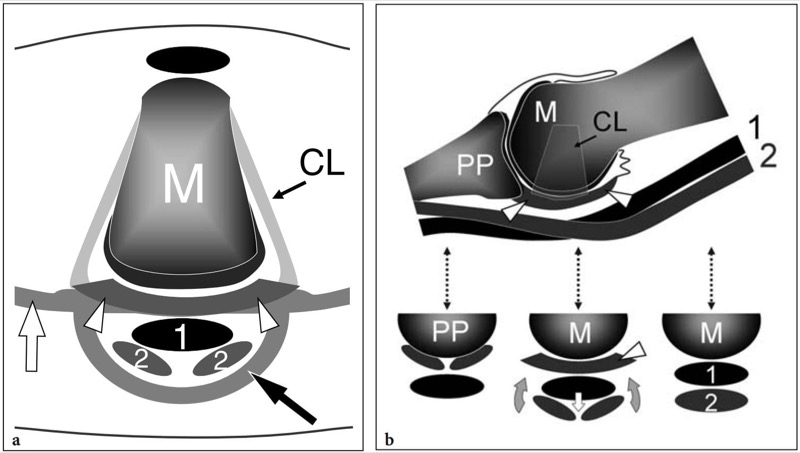
The joints of the lesser toes are the metatarsophalangeal and the proximal and distal interphalangeal joints. These are synovial-lined joints that allow flexion-extension movements of the toes. Similar to the metacarpophalangeal joints, thick fibrocartilaginous plantar plates insert into the base of the proximal phalanx and extend posteriorly to cover the cartilage of the plantar aspect of the metatarsal heads (Fig. 5). The plantar plates serve as the weight-bearing platform of the metatarsal heads and are the main stabilizers of the metatarsophalangeal joints by resisting dorsiflexion (Mohana-Borges et al. 2003; Blitz et al. 2002, 2004). Any compromise to their integrity creates instability of the joints (Blitz et al. 2004). The flexor digitorum longus and flexor digitorum brevis tendons run on the inferior aspect of the plantar plates inside a common fibrous sheath invested by a synovial membrane. The sheath is composed of the anterior insertion of the plantar fascia and by its transverse fibers. Because the plates are interposed between the tendons and the joint spaces, a full-thickness tear in this region causes a communication between the articular cavity and the tendon sheath. The plantar plates are connected on each side with the collateral ligaments – which are strong fibrous bands that allow limitation of adduction and abduction – and inferiorly with the intermetatarsal ligament (Fig. 5b). The collateral ligaments are fan-like intra-articular structures which attach to the epicondyles of the metatarsal neck and create the medial and lateral walls of the fibrous capsule. The deep transverse intermetatarsal ligament attaches to the medial and lateral aspects of the plantar plate (Fig. 5a). Dorsally, the expansion of the extensor digitorum longus and brevis tendons forms the roof of the joint.
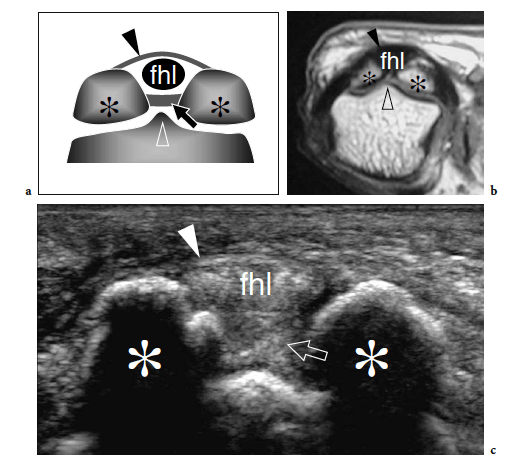
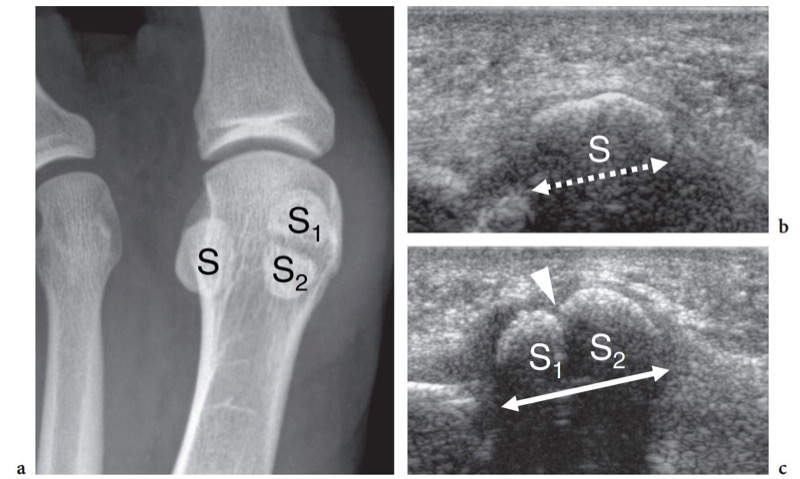
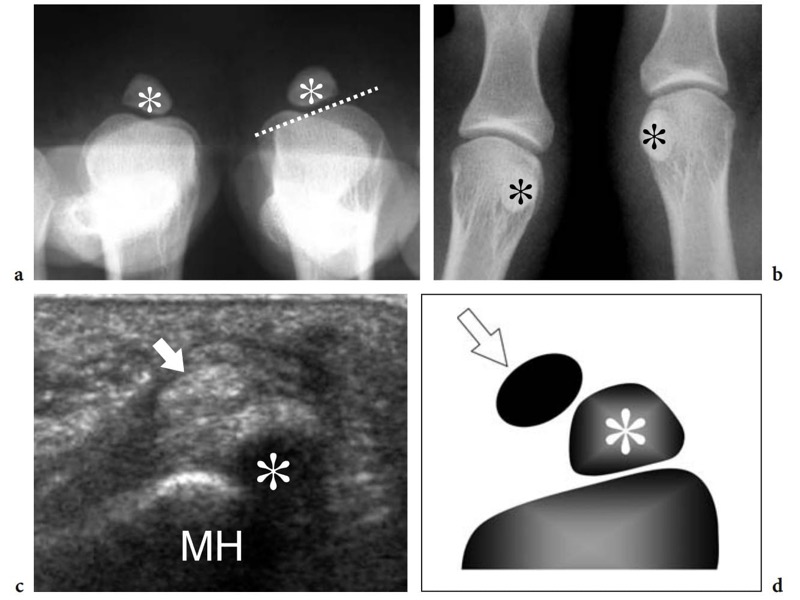
The great toe has two phalanges only – the proximal and the distal – and one interphalangeal joint. Two sesamoid bones – the medial (tibial) and the lateral (fibular) – are found at the plantar aspect of the metatarsophalangeal joint, facing the plantar aspect of the first metatarsal head (Fig. 6a,b). Sesamoids are embedded in the medial and lateral tendon slips of the flexor hallucis brevis muscle and in the tendon of the abductor hallucis muscle (Jahss 1981). They are interconnected by a thick intersesamoid ligament which acts as a reflection pulley for the flexor hallucis longus tendon. Based on their critical position in the capsuloligamentous sling of the metatarsophalangeal joint, sesamoids provide mechanical benefit during joint flexion. In addition, they participate in absorbing weightbearing stress, in protecting the tendons of the flexor hallucis longus and brevis, and in reducing the friction between them and the underlying joint (Jahss 1981). The size and shape of the sesamoids is variable. Most of the attention in the literature has been focused on their partition (Jahss 1981; Karasick and Schweitzer 1998). In a series of 200 feet, bipartite sesamoids were described in 13.5% of cases with 37% bilaterality (Prieskorn et al. 1993) (Fig. 7a). Radiographic nonvisualization of one or both sesamoids is exceedingly rare, only 12 cases being reported in the literature with 3 patients showing absence of the fibular sesamoid (Jahss 1981; Karasick and Schweitzer 1998; Clifford et al. 1998; Le Minor 1999) (Fig. 8a–d). Several hypotheses had been proposed to explain this condition. Some authors postulate that this anomaly reflects an evolutionary phenomenon related to the tendency toward disappearance of the sesamoid bones within hominoid primates, probably related to genetic factors (Le Minor 1999). Others suggest that their nonvisualization can be related to a developmental anomaly related to defective ossification.
Differential diagnosis of absent sesamoids includes infection and surgical removal (Brock and Meredith 1979). Although this condition is usually painless, some authors have reported painful callosities, hammer toe deformities, and possibly hallux valgus as causes of local mechanical derangement. In addition to the hallux sesamoids, a small sesamoid can be found at the plantar aspect of the fifth metatarsophalangeal joint. In hallux valgus, a shift in tendon alignment at the first metatarsophalangeal joint occurs with the insertion of the abductor hallucis tendon that becomes plantarward and the flexor and extensor tendons that bowstring at the first metatarsophalangeal joint. This shift contributes to development of the deformity. Similar to MR imaging, US can demonstrate changes in the tendon route (Eustace et al. 1996).
In ambulatory medical practice, foot pain is one of the most common musculoskeletal complains. It can cause significant discomfort and disability, limiting not only the athletic performance in sportsmen but also daily activities in sedentary patients. A wide spectrum of foot-specific disorders involving soft tissues, bones and joints, nerves and vessels, as well as a variety of systemic diseases, can cause foot pain.
A detailed history of the patient and a careful physical examination are of the utmost importance in narrowing the list of differential diagnoses. Systemic inflammatory arthritis and spondyloarthropathies, previous local trauma, a sudden increase or change in training strategies for sportsmen, and the exact location and type of pain (burning or tingling would suggest nerve entrapment, night pain an inflammatory condition, exercise-related pain a tendinopathy or degenerative joint disease, etc.) should be carefully assessed. Then, a complete physical examination must include evaluation of the skin and subcutaneous tissue, neurologic and vascular assessment and, finally, analysis of the musculoskeletal structures. While taking the history we usually perform a brief local examination (including inspection, palpation, and a general evaluation of the range of movements) targeted to the area of maximal pain. When a nerve lesion is suspected, examination of the tactile sensitivity is performed. In any case, a focused clinical question in the examination request (e.g., Is there a Morton neuroma in the third web space? Is there a radiolucent foreign body on the plantar aspect of the heel? Can you inject steroids on the plantar fascia insertion?) is helpful, at least to reduce the examination time. In patients with heel pain, the bony prominences of the calcaneus are palpated in order to reveal tenderness or palpable defects. Patients with plantar fascia enthesopathy complain of localized pain over the inferomedial aspect of the calcaneus, at approximately 3-4 cm from the posterior heel. Pain is sharp and most severe with the first step out of the bed in the morning or after prolonged rest. Stress fractures of the calcaneus present with prolonged and invalidating pain at the inferior, medial, and lateral aspect of the heel. Often, patients indicate the location of pain by pinching the calcaneus between the thumb and the fingers. Pain related to plantar vein thrombosis is referred more anteriorly than enthesopathy and shares similar characteristics with plantar fasciitis. The anterior portion of the middle cord of the plantar aponeurosis must be carefully palpated to rule out soft-tissue masses related to Ledderhose disease. Benign tumors and tumor-like conditions represent most of the soft-tissue masses in the foot. Because of their uncommon occurrence, malignant tumors are often unsuspected and misdiagnosed clinically, especially if they occur in young individuals with nonspecific or longstanding clinical symptoms (Woertler, 2005). In the lower limb, the foot is the preferred site for growth of ganglion cysts (Rozbruch et al. 1998). Ganglia most often involve the hindfoot and the midfoot and present as firm well-circumscribed lumps which are not fixed to the overlying skin. The sheaths of extrinsic foot tendons should be palpated to rule out tenosynovitis.
Many of these tendons can be assessed by asking the patient to perform resisted movements against the examiner’s hand (i.e., the extensor hallucis longus is easily evaluated by asking the patient to dorsiflex the distal phalanx of the great toe against the examiner’s thumb). Forefoot pain due to arthritis of the metatarsophalangeal joints present with local swelling, tenderness, and pain exacerbated by flexion-extension movements of the affected toes. In rheumatoid arthritis, early bone erosions typically involve the fifth metatarsophalangeal joint, whereas seronegative spondyloarthropathies more commonly affect the tendon enthesis and the synovial joints of the forefoot (Brook and Corbet 1977; D’Agostino et al. 2003; Borman et al. 2005). In psoriatic arthritis, a single toe is affected: it presents as markedly swollen – so-called “sausage toe” – as a result of the inflammatory process that involves the metatarsophalangeal and the interphalangeal joints.
Morton neuroma can be found in all intermetatarsal spaces but is more often encountered between the heads of the third and fourth metatarsals, probably because of the smaller size of the web space and the more fixed position of the interdigital nerve. The patient (most often a middle-aged woman) refers local sharp pain at the base of the web space radiating to the toes. Pain is worsened by wearing shoes and walking, and can be so excruciating that some patients are compelled to take their shoe off to alleviate it. Squeezing the forefoot while applying firm pressure over the plantar aspect of the involved space can cause entrapment of the neuroma between the metatarsal heads, thus reproducing the patient’s pain. The Mulder sign can be obtained when the examiner holds the first, second, and third metatarsal heads with one hand and clutches the fourth and fifth ones with the other. By pushing the medial foot up and the lateral foot down, the examiner can cause dislocation of the neuroma with a resultant palpable click (Mulder 1951). An alternative way to perform the Mulder test is to clasp the metatarsal heads with the left hand while the thumb of the right hand exerts pressure on the sole of the foot at the point where the neuroma is suspected. Apart from the Mulder sign, forced dorsiflexion of the toes can stretch the interdigital nerve and reproduce the patient’s pain (Lasegue sign for Morton neuroma). Compression of the web space between the index finger (from above) and the thumb (from below) can also trigger pain (Tinel sign). In metatarsalgia, pain is referred to the plantar aspect of a metatarsal and may be associated with loss of concavity of the transverse arch leading to secondary increased pressure on the second and third metatarsal heads. This condition is often associated with toe deformities, including hallux valgus, claw toe (hyperextended metatarsophalangeal joint, flexed proximal and distal interphalangeal joints), and hammer toe (hyperextended metatarsophalangeal joint, flexed proximal interphalangeal joint, and extended distal interphalangeal joint).
Local pressure exerted on the plantar aspect of the metatarsal head may reproduce pain. Hammer toe deformity commonly involves the second toe and is frequently associated with hallux valgus. As a result of mechanical irritation, painful corns appear on the dorsal aspect of the proximal interphalangeal joint and tender callosities develop under the corresponding metatarsal head. Insufficiency (stress) fractures most commonly affect the second metatarsal neck but can also involve the base of the fourth and fifth metatarsals and the neck of the fourth metatarsal. The location of these tears probably depends on an altered distribution (hallux deformities, pes cavus, etc.) of the load on the metatarsals. The clinical diagnosis may be difficult because patients refer pain over the metatarsophalangeal joint even if the fracture affects the metatarsal neck. Pain can be reproduced with pressure over the dorsal aspect of the metatarsal head while stabilizing the metatarsal base with the other hand.
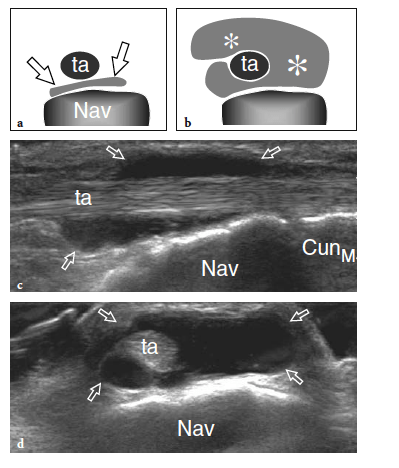
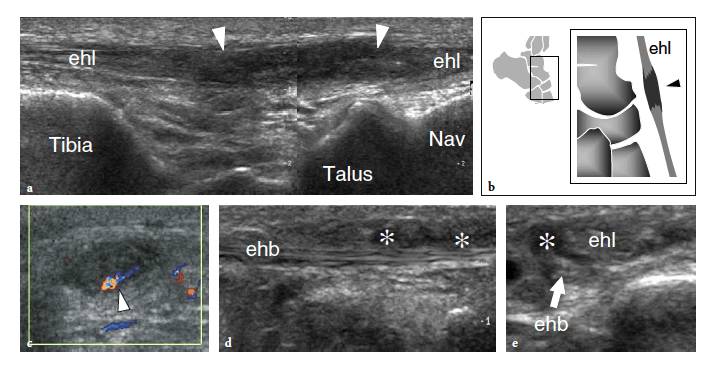
The standard US examination of the foot begins with its dorsal aspect, keeping the patient supine with the knee flexed at approximately 90°. The sole of the foot lies on the examination bed while the ankle is in slight plantar flexion. Transverse US imaging planes are the best suited to identify the superficial long tendons as they course over the dorsum of the foot. The most medial tendon is the tibialis anterior, which gradually tapers as it runs toward the medial border of the foot to insert on the anteromedial aspect of the medial cuneiform and the base of the first metatarsal (Fig. 9). One should remember that the distal portion of this tendon is medial and not dorsal as expected, and may show a division prior to insertion that represents a normal variant and not a longitudinal split of the distal tendon (Mengiardi et al. 2005). In a more medial position, the extensor hallucis longus tendon is found (Fig. 10a). It is a thin tendon and can be more easily detected during passive flexion and extension movements of the great toe. The four diverging slips of the extensor digitorum longus muscle for the lesser toes can be detected in a more lateral position. High-frequency probes may be necessary to clearly depict these very small and superficial structures.
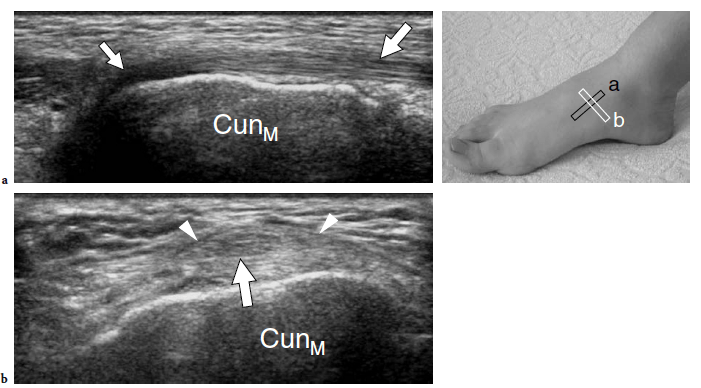
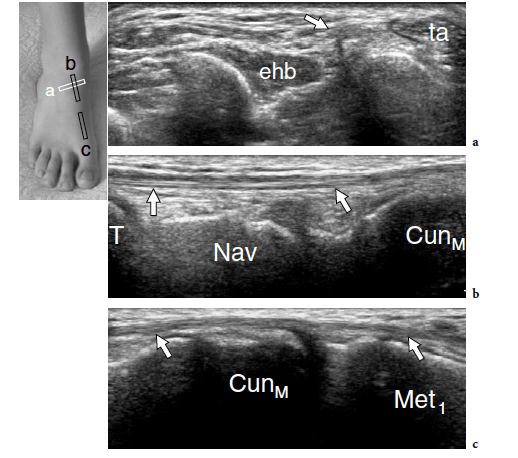
Occasionally, the peroneus terzius can be appreciated as an accessory fifth lateral slip of the extensor digitorum longus directed toward the base of the fifth metatarsal. The extensor hallucis brevis and extensor digitorum brevis muscles lie just deep to the diverging slips of the extensor digitorum longus. In many cases, these two muscles cannot be separated because they have a common muscle belly. The extensor brevis muscles can be seen arising from the lateral aspect of the calcaneus and ending in the respective distal tendons. Careful scanning with a high-resolution transducer can demonstrate the extensor digitorum brevis tendons inserting into the lateral aspect of the respective tendons of the extensor digitorum longus. The extensor tendons can be imaged up to their distal insertion on the phalanges (Fig. 11).
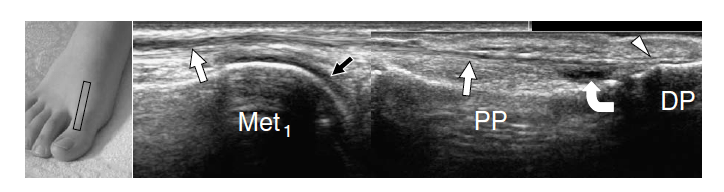
The dorsalis pedis artery and the medial branch of the deep peroneal nerve can easily be detected over the anterior ankle using transverse planes and then followed down to reach the metatarsal region (Fig. 12). The artery is a useful landmark to identify the nerve. The joint recesses and the bones of the dorsal foot are better delineated on longitudinal planes. Sagittal US images over the ankle joint allow detection of the dorsal aspect of the talus and the anterior ankle recess, the navicular bone with the dorsal talonavicular joint, and the cuneiforms. Over the cuneiform area, shifting the transducer in the transverse plane makes distinction of the individual cuneiforms and the intercuneiform joint spaces easier. More distally, the transducer should be turned again in the sagittal plane to evaluate the tarsometatarsal joint with the medial metatarsals, and the joints of the medial toes. Sagittal scanning over the lateral midfoot allows identification of the calcaneocuboid joint together with the dorsal calcaneocuboid ligament, the fourth and fifth metatarsals, and the distal joints of the lateral lesser toes.
The tarsal joints must be carefully assessed to rule out synovial effusions, synovial hypertrophy, marginal bone erosions, and ligament discontinuity. It should be noted that a small effusion in the dorsal recesses of the metatarsophalangeal or interphalangeal joints is a normal findings and should not be misinterpreted as a sign of synovitis.
The standard US examination of the plantar aspect of the foot is performed with the patient supine and both his/her legs on the bed or placed on a pillow to obtain a more comfortable position. Sagittal images obtained slightly medial to the midline axis of the foot are first obtained over the calcaneal tuberosity to image the preinsertional portion of the plantar fascia. This appears as a distinct thick hyperechoic fibrillar band, somewhat similar to a tendon, running parallel to the skin of the sole (Fig. 13a,b). At the level of insertion, the most posterior fibers of the fascia course obliquely from surface to depth relative to the transducer position and may appear falsely hypoechoic as a result of anisotropy (Fig. 13b). Slight tilting of the transducer can resolve this artifact. Then, the transducer is swept distally to follow the fascia, which becomes progressively thinner and superficial as it proceeds toward the forefoot (Fig. 13c). The strong central cord of the plantar fascia lies over the surface of the thick muscle belly of the flexor digitorum brevis (Fig. 13c). In normal states, it is approximately 3–4 mm thick (Cardinal et al. 1996; Gibbon and Long 1999; Walther et al. 2004). Shifting the transducer in a more lateral position allows assessment of the thinner external part of the plantar fascia that overlies the abductor digiti minimi muscle. An accurate scanning technique may help to improve the separation of the fascia from the deep muscles. Transverse planes over the plantar fascia may be useful to show the relationships of the central cord with the deeper structures as well as the distal splitting of the fascia (Fig. 14). The medial cord of the aponeurosis, which is located inferior to the abductor hallucis muscle, appears as the thinnest portion.
In normal conditions, blood flow signals are not visible at the enthesis of the plantar aponeurosis with power Doppler imaging and contrast-enhanced US (Morel et al. 2005).
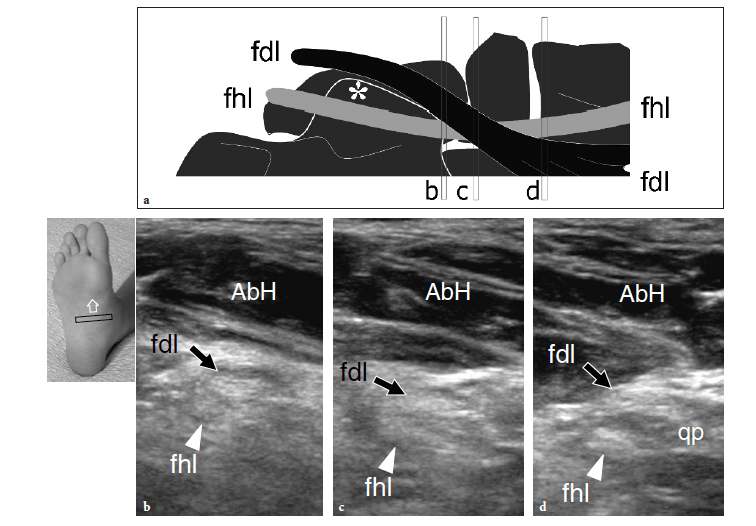
The abductor hallucis muscle can easily be examined from its posterior origin through to its distal insertion by means of coronal and oblique transverse planes. In a deeper location, the second muscle layer containing the quadratus plantae muscle, the lumbricals, and the tendons of the flexor hallucis longus and flexor digitorum longus is visualized (Fig. 14). Scanning during active and passive flexion and extension movements of the great and lesser toes can aid their identification. The sustentaculum tali is readily identified as a large bony prominence of the medial aspect of the calcaneus. It represents a useful landmark to identify the flexor digitorum longus tendon – which passes superficial to it – and the flexor hallucis longus tendon – which travels along its undersurface (Fig. 15a). Coronal planes are adequate for this purpose (Fig. 15b).
Due to problems of access, US has intrinsic difficulties assessing the spring ligament, which courses from the sustentaculum tali to the navicular tubercle. More distally, in the sole, the flexor hallucis longus crosses the tendon of the flexor digitorum longus, to which it is connected by a thin fibrous slip (Fig. 16a). Sweeping the probe from posterior to anterior on short-axis planes is essential to correctly image the long flexors in this area (Fig.16b–d). Small amounts of sheath fluid may help the distinction between them. Using a plantar approach, the flexor digitorum longus tendon is the most superficial. Distal to the crossing point, this tendon can be seen receiving the insertion of the quadratus plantae (Fig. 16d). The medial and lateral plantar neurovascular bundles course in close relationship with the long flexors and can be recognized on short-axis planes (Fig. 15b). On the lateral aspect of the sole, the peroneus brevis continues its straight course just deep to the subcutaneous tissue up to reach the base of the fifth metatarsal (Fig. 17a). On the other hand, the peroneus longus assumes an oblique course from surface to depth as it approaches thecuboid (Fig. 17b). A careful scanning technique is required to image the peroneus longus in this area due to anisotropy, as well as to assess the presence of an accessory ossicle, the so-called os peroneum, within its substance. Then, using a plantar approach, the peroneus longus tendon can be seen leaving the cuboid tunnel to run obliquely across the sole up to insert into the first metatarsal (Fig. 17c). Scanning the metatarsal region allows detection of the interosseous muscles located among the metatarsals and assessment of the extrinsic tendons.
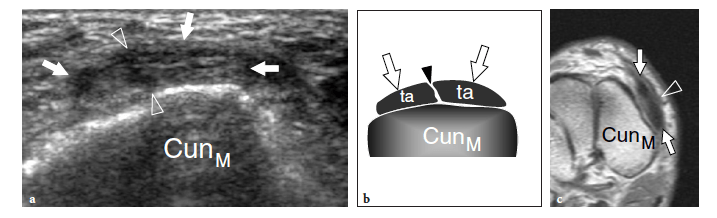
Transverse US images obtained over the metatarsophalangeal joint of the great toe show two sesamoids as paired oval hyperechoic structures with posterior acoustic shadowing (Fig. 6c). The flexor hallucis longus tendon is held in between them: this position prevents tendon damage against the ground during the step-off phase of walking. At US examination, a bipartite sesamoid appears as a bony complex formed by two distinct ossicles invested by a cortical layer. Typically, bipartite sesamoids have a larger size and exhibit rounded borders: this latter sign may aid in distinguishing an anatomic variant from an acute fracture. Local pressure with the probe may help the diagnosis, but the possible occurrence of a painful bipartite sesamoid should be taken into account (Frankel and Harrington 1990). Sesamoid aplasia is readily manifest at US examination. It appears as visualization of one ossicle associated with a flat appearance of the plantar articular face of the metatarsal and a slightly subluxed flexor hallucis longus tendon. Long-axis and short-axis US images over the inferior aspect of the metatarsophalangeal joints reveal the plantar plates as triangular flexible structures. Being fibrocartilaginous in nature, they appear homogeneously hyperechoic (Fig. 18). Plantar plates can be also observed at the interphalangeal levels.
A variety of disorders can involve the soft tissues around the foot. They are here reviewed by location, dividing the foot into three regions: the hindfoot and midfoot examined in their dorsal and plantar aspects, and the forefoot.
Tibialis Anterior and Extensor Tendon Abnormalities
A recent study showed that degenerative changes in this tendon occur distally, within 3 cm of the insertion on the anteromedial aspect of the medial cuneiform and the base of the first metatarsal (Mengiardi et al. 2005). Tendinosis presents with increased tendon thickness, echotextural abnormalities, intratendinous focal hypoechoic areas, and irregularities in the underlying bones. A careful scanning technique may be needed to distinguish distal longitudinal splits from a bifid tendon appearance that represents a normal variant.
Fissurations may be suspected only when the tendon insertion is markedly thickened and hypoechoic (Fig. 19). Similar to the distal biceps tendon, the distal tibialis anterior is not invested by a synovial sheath but it is separated from the dorsal cortex of the navicular and the first cuneiform by a synovium-lined bursa that reduces friction of the tendon over the bone during walking. If inflamed by local attrition, this bursa appears as a hypoechoic structure that partially surrounds the tendon, mimicking tenosynovitis (Fig. 20).
Extensor hallucis longus and extensor digitorum longus tendon tears are usually secondary to direct trauma against the dorsum of the foot. The clinical diagnosis is based on the patient’s inability to extend the toes. In a preoperative setting, US may be useful to assess the grade of retraction of the torn tendon ends (Fig. 21).
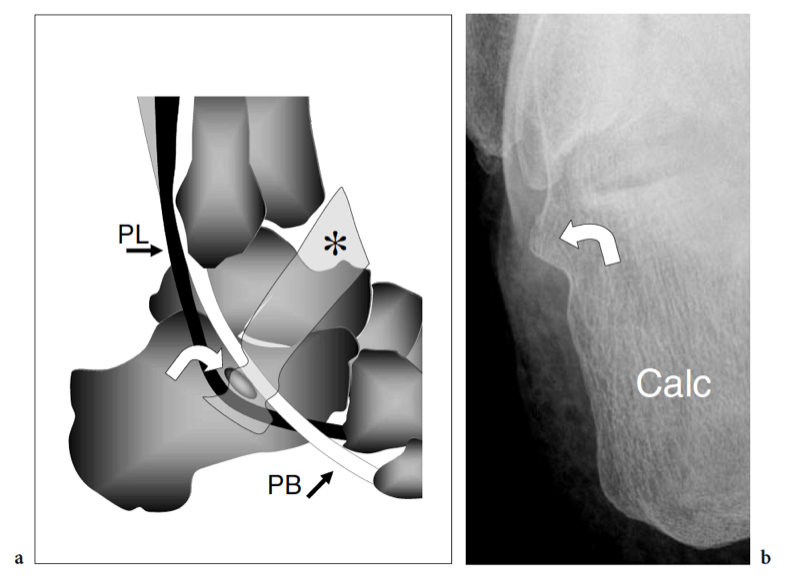
After reflecting behind the lateral malleolus, the peroneal tendons reach the inframalleolar area, running alongside the lateral aspect of the calcaneus. The lateral aspect of the calcaneus has two bony prominences: the peroneal tubercle – which is also referred to as the peroneal trochlea – and the retrotrochlear eminence – into which the peroneus quartus inserts.
Relative to the peroneal tubercle, the peroneus brevis courses superiorly and the peroneus longus inferiorly (Fig. 22). Both tendons are retained against the calcaneus by the inferior peroneal retinaculum that inserts into the tip of the tubercle. Anatomic variants of the peroneal tubercle are not infrequent (Hyer et al. 2005a; Wang et al. 2005). The tubercle can be either hypoplastic or completely absent or it may be larger and abnormal in shape (Fig. 23).
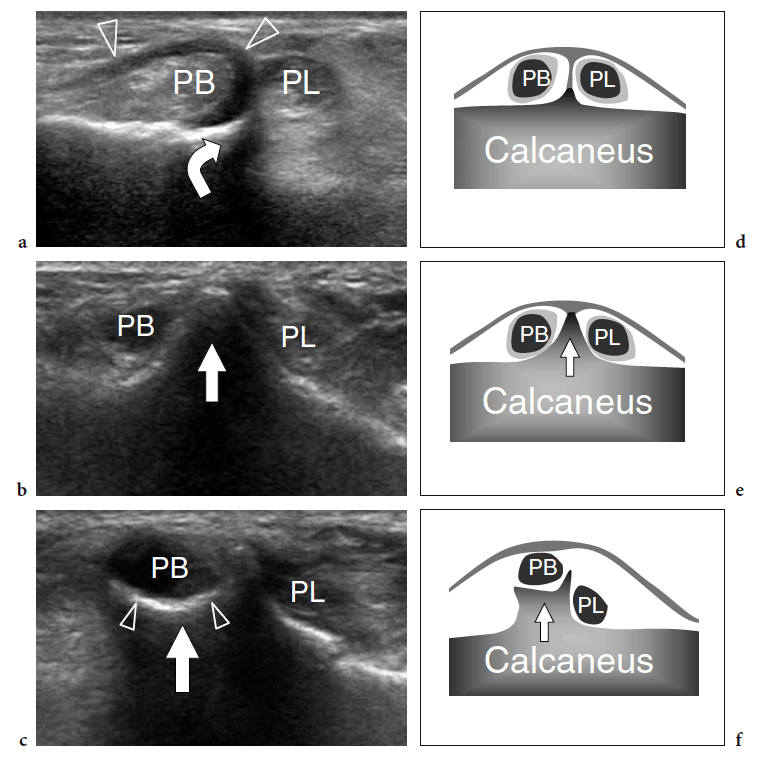
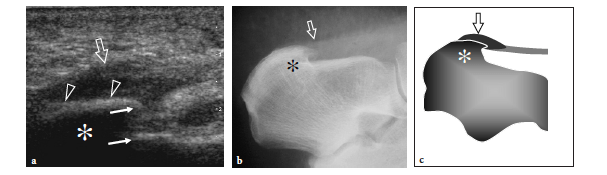
In an anatomic study on the peroneal tubercle morphology performed in a large series of bones from an osteologic collection, this tubercle appeared flat in 42.7%, prominent in 29.1%, concave in 27.2%, and tunnelized in 1.0% of cases (Hyer et al. 2005a). A markedly enlarged tubercle – a condition which is commonly known as hypertrophied tubercle or osteochondroma of the peroneal tubercle – has clinical relevance because it may cause impingement on the peroneal tendons leading to stenosing tenosynovitis or chronic friction of these tendons against the anomalous tubercle during walking (Pierson and Inglis 1992; Martin et al. 1995; Bruce et al. 1999). Tubercle hypertrophy seems to derive from altered weight-bearing and inflammatory changes related to peroneus longus spasm (Boles et al. 1997). Clinically, patients present with a firm, painless lump on the lateral aspect of the calcaneus, located just under the tip of the lateral malleolus, related to the enlarged tubercle and chronic irritation of superficial soft tissues against the shoe. If tenosynovitis is present, the tender, inflamed peroneal tendon sheath can become palpable. Axial radiographs of the calcaneus can easily show a hypertrophied tubercle. US is able to demonstrate the enlarged tubercle and can easily assess its characteristics. The tubercle appears as a bony prominence on the lateral aspect of the calcaneus that most often shows a pointed or concave shape.
A careful scanning technique is required to identify the peroneal tendons, to evaluate their relationships with the abnormal tubercle, and to assess the tendon echotexture and rule out possible tenosynovitis or tears. CT and MR imaging have a value in a preoperative setting. Tubercle resection is indicated in symptomatic patients who do not respond to conservative therapy. Surgery leads to complete recovery (Martin et al. 1995).
Distal to the peroneal tubercle, the peroneus longus tendon reflects under the inferomedial border of the cuboid to proceed toward the base of the first metatarsal. A smooth bony sulcus exists on the plantar surface of the cuboid at the level of which the tendon redirects its course to enter the solex. In this area, a sesamoid bone – the os peroneum – can be seen within the peroneus longus with a prevalence ranging from 5% to 26% (Le Minor 1987; Kruse and Chen 1995). This ossicle shows high variability in shape and size and is often bipartite or multipartite. (Figs. 24, 25). Although the os peroneum can fracture as the result of excessive chronic loading (Okazaki et al. 2003) or acute direct trauma, the most common mechanism of fracture is a violent contraction of the peroneus longus muscle in response to a sudden ankle sprain in inversion or supination (Peacock et al. 1986; Wander et al. 1994; Sobel et al. 1994; Bianchi et al. 1991; Brigido et al. 2005). In these patients, the clinical diagnosis of os peroneum fracture is challenging because signs and symptoms resemble those related to ankle sprains or a tendon abnormality. Radiographically, the fracture of an os peroneum can be confused with a bipartite or multipartite ossicle: this may lead to a delayed diagnosis and possible sequelae, including ankle instability and peroneal compartment syndrome (Brigido et al. 2005). The diagnosis of an os peroneum fracture basically relies on standard radiographs that show multiple fragments with absence of sclerotic borders, separated from one other by more than 6 mm (Brigido et al. 2005). Follow-up radiographs are able to detect the progressive retraction of the posterior fragment(s), indicating a tear of the peroneus longus tendon (Tehranzadeh et al. 1984; Bianchi et al. 1991) (Fig. 26). US can help the diagnosis if standard radiographs are equivocal. Typically, the fractured fragments appear more irregular in shape compared with a multipartite ossicle. The gap between the fragments can easily be measured on long-axis planes by comparing the results of serial US studies. When substantial separation is present, the absence of fibrillar echotexture between the fragments indicates a tear of the peroneus longus. Local pain related to transducer pressure may help to differentiate bipartite ossicles from fractures. MR imaging shows local edema and dislocation of the proximal fragment, although the individual fragments can be difficult to visualize because of their small size and local bone marrow edema. An os peroneum fracture seems better evaluated using radiography and US rather than radiography and MR imaging (Brigido et al. 2005).
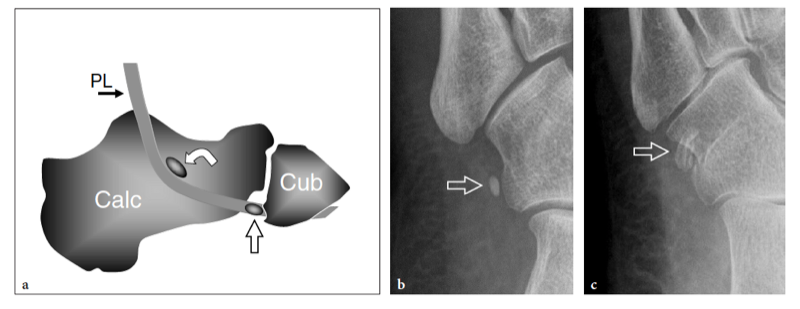
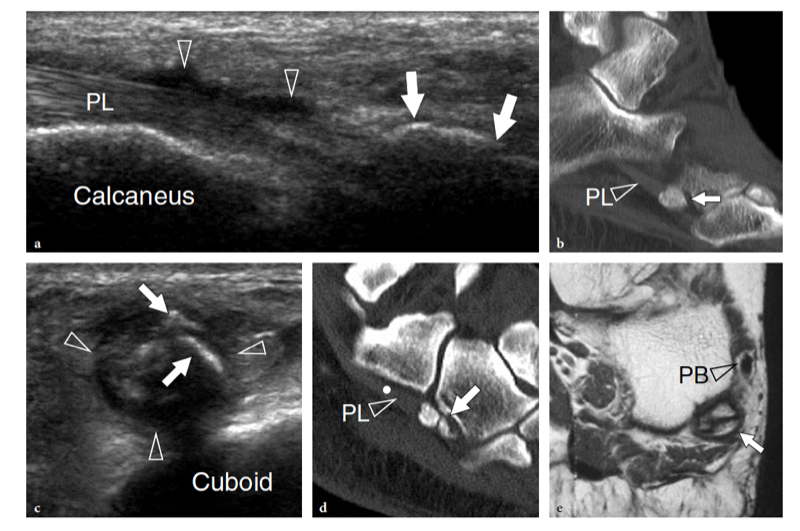
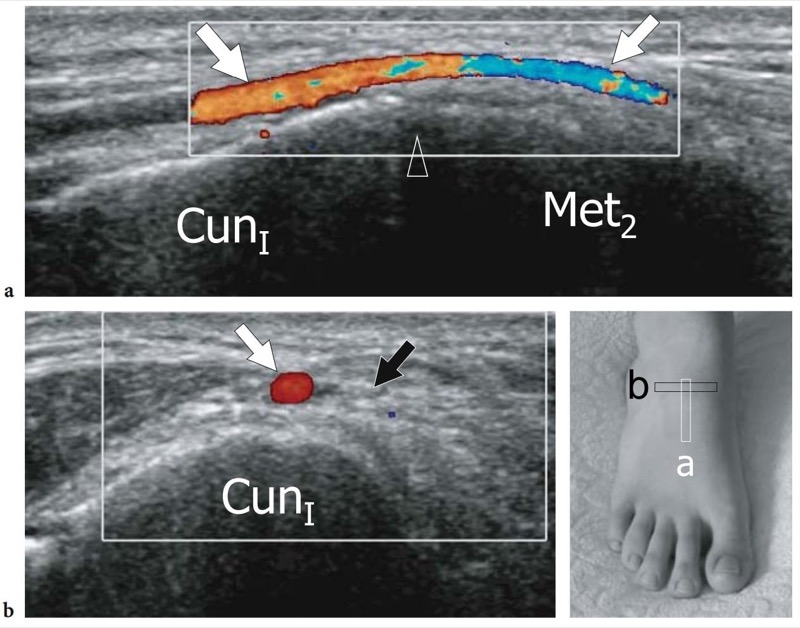
Entrapment of the deep peroneal nerve at the dorsal aspect of the hindfoot and midfoot may occasionally be encountered in runners, soccer players, skiers, and dancers (Schon 1994; McCrory et al. 2002; Delfaut et al. 2003). The nerve can be compressed at several locations, including the point under the superior edge of the inferior extensor retinaculum, where the extensor hallucis longus tendon crosses over it, and the area underneath the tendon of the extensor hallucis brevis. Osteophytes of the talonavicular joint, navicular-cuneiform joints, or cuneiform-metatarsal joints have also been implicated. Recurrent ankle sprains are predisposing to this condition. In these cases, the nerve is placed under maximum stretch over the dorsal capsule of the joint, as the foot plantar flexes and inverts. Soccer players receiving repetitive blows over the dorsum of the foot while kicking the ball, ballet dancers who have prominent dorsal ridges of the tarsal joints, and skiers with tight-fitting ski boots have been known to develop this neuropathy (Schon 1994). In these cases, the patient experiences burning pain radiating down to the dorsum of the foot with elective pinpoint tenderness (Tinel sign) at the level of the nerve lesion. US is able to image the deep peroneal nerve by sweeping the probe from the epiphysis of the distal tibia downward. At the dorsal aspect of the ankle, the deep peroneal nerve can easily be recognized on transverse planes superficial to the tibia and adjacent to the anterior tibial artery and vein. It typically crosses the tibialis anterior artery, passing from medial to lateral. At the site of injury, progressive swelling of the nerve fascicles can be observed with US as a result of trauma. (Fig. 27).
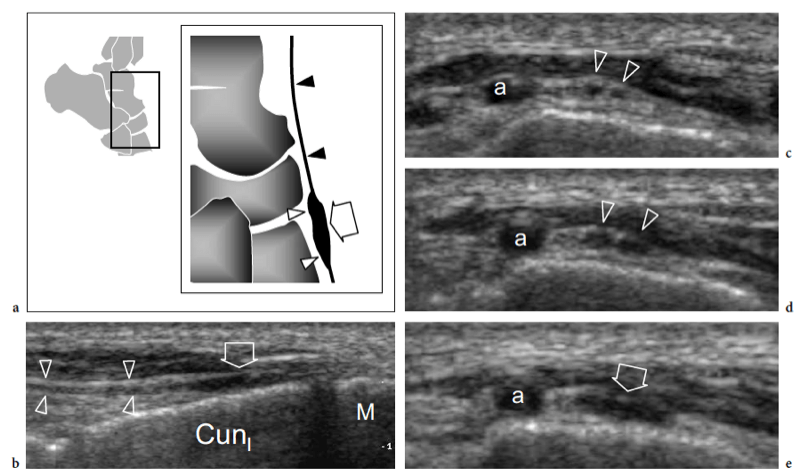
Fig. 27a–e. Deep peroneal neuropathy. a Schematic drawing of the dorsal midfoot with the ankle in plantar flexion illustrates the mechanism of deep peroneal neuropathy related to compression of the nerve (black arrowheads) against the underlying bones by external forces (arrow). A spindle neuroma (white arrowheads) may develop from the injured nerve fascicles at the site of trauma. b Long-axis 12-5 MHz US image of a 32-year-old soccer player who had received repetitive blows to the dorsum of the foot shows focal hypoechoic thickening (arrow) of the deep peroneal nerve (arrowheads) at the point where the nerve is closely apposed to the fl -at dorsal surface of the middle cuneiform (CunI). M, metatarsal. c–e Serial 12–5 MHz US images obtained from c proximal to e distal over the short axis of the injured nerve reveal progressive swelling of the fascicles (arrowheads) evolving into a neuroma (arrow). a, dorsalis pedis artery. In the diagram, the insert at the upper left indicates the area of interest
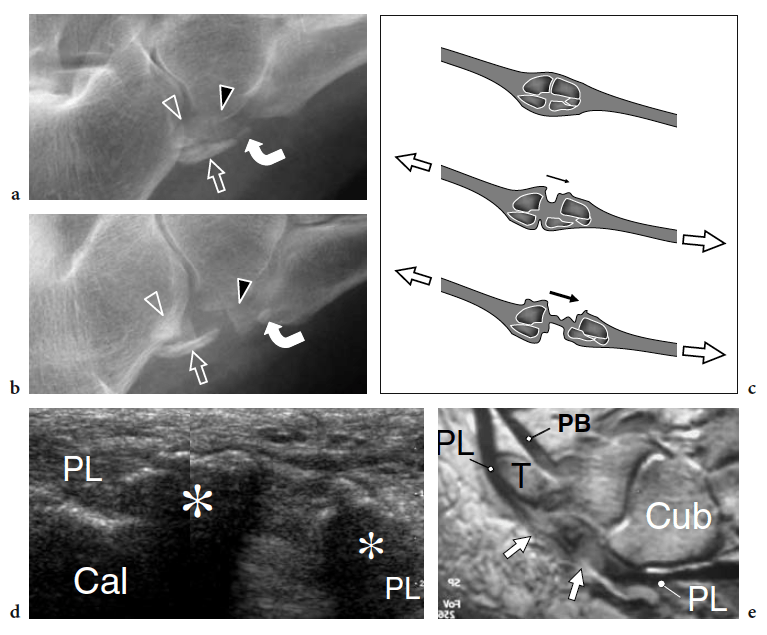
Injuries of the dorsal midtarsal ligaments are commonly observed as a result of ankle sprains. Excessive traction trauma can result in their intrasubstance rupture or bone avulsion at their insertion into bone. Ligament tears are demonstrated as thickening and an irregular hypoechoic appearance of the affected structure. Often, tarsal ligaments are affected as part of more extensive trauma, involving the ankle ligaments. Differentiation between partial and complete tears is difficult on the basis of the US findings alone. The cortical surface of the tarsal bones must be accurately assessed during routine US scanning because the identification of subtle cortical avulsions or fractures may not be straightforward on routine radiographs. On the other hand, bone abnormalities can occasionally be recognized during a US examination performed for soft-tissue assessment.
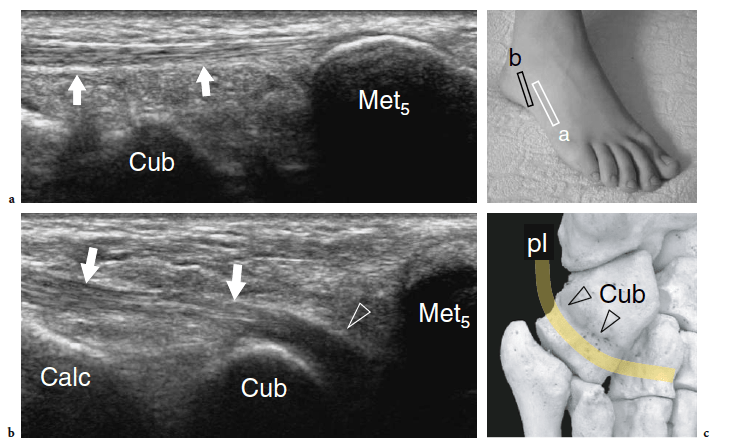
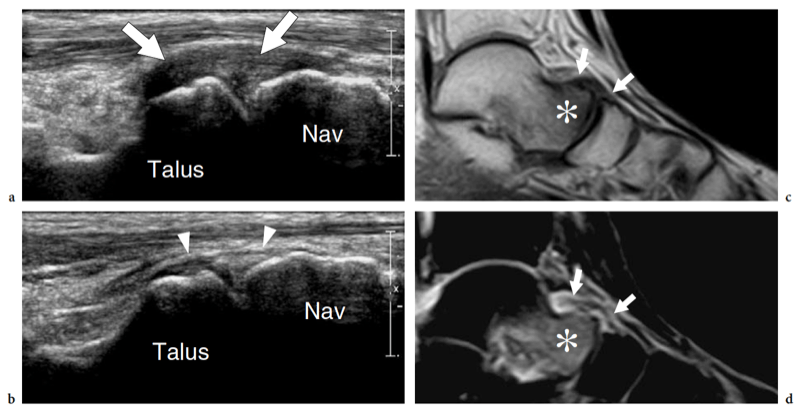
Some midtarsal ligaments are particularly vulnerable to strain injuries. In forcible plantar strains, the dorsal capsule of the talonavicular joint and the dorsal talonavicular ligament can rupture. The dorsal aspect of the talar head and the navicular may undergo avulsion of bone fragments related to detachment of ligament insertions. In intrasubstance rupture, long-axis US images show the injured ligament as a thickened hypoechoic structure bulging over the dorsal capsule (Fig. 28). In the acute phase, a hyperemic blood flow pattern can be identified at Doppler imaging. One-to-one comparison with the contralateral foot may be helpful to confirm subtle abnormalities. In trauma with cortical avulsion, US reveals the detached fragment as a hyperechoic structure that is displaced dorsally, leaving a defect in the bone (Fig.29). In ankle inversion sprains, similar findings can be observed in the dorsal calcaneocuboid ligament that is located on the lateral border of the midfoot. This ligament may tear without or with involvement of the lateral ligamentous complex of the ankle, including the anterior talofibular ligament and the calcaneofibular ligament. Because of its small size, the avulsed fragment is difficult to detect on standard radiographs and can easily go unnoticed. US reveals the avulsed fragment as a hyperechoic structure connected with the ligament and the extensor digitorum brevis muscle, allowing evaluation of its size and displacement (Fig. 30).
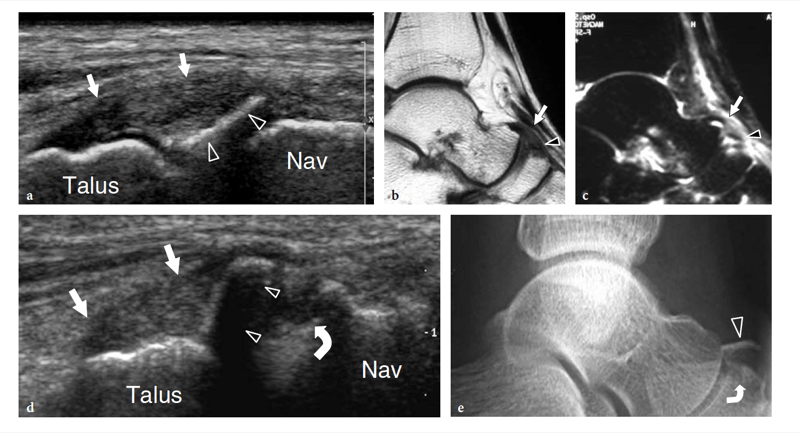
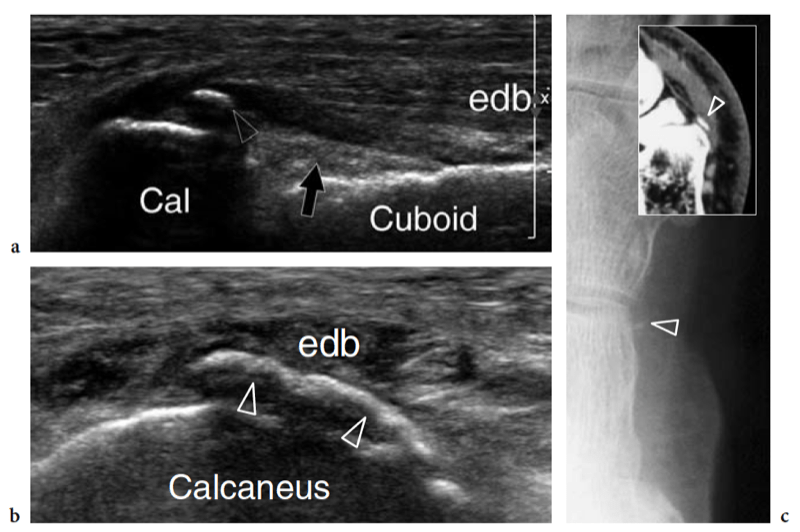
Recently, the US appearance of anterosuperior calcaneal process fracture has been described (Boutry et al. 2006). This lesion may be secondary to either avulsion of the origin of the calcaneocuboid component of the bifurcate ligament, resulting from excessive inversion and plantar flexion of the foot, or impaction of the anterior aspect of the calcaneus against the cuboid, derived from excessive eversion and dorsiflexion of the foot. The avulsed bony fragment is usually larger than that observed in the avulsion of the dorsal calcaneocuboid ligament and may be associated with injury to the talonavicular ligament (Chopart sprain). Although the diagnosis can be made on standard radiographs, anterosuperior calcaneal process fractures can easily be missed if an internal oblique view of the tarsal region is not obtained. Knowledge of the US appearance of these fractures may allow recognition of radiographically occult lesions and may contribute to establish a proper treatment and avoid painful nonunion. CT and MR imaging can be performed for more accurate evaluation if surgical repair is needed (Meyer et al. 1988; Robbins et al. 1999). Finally, US has proven to be an effective tool for detecting occult fractures of the foot, including the base of the fifth metatarsal and the cuboid (Enns et al. 2004; Dudkiewicz et al. 2005; Wang et al. 1999).
Arthritis involving the tarsal joints is most often encountered in patients affected by rheumatoid arthritis, spondyloarthropathies, or neuropathic joint disease. All these conditions cause local pain and deformity, leading to impairment of standing and walking. As a rule, in the course of rheumatoid arthritis, changes in the hindfoot and midfoot occur later than in the forefoot. US shows intra-articular synovial fluid as a thin hypo-anechoic collection located within the joint cavity because the synovial recesses of the midtarsal joints are usually too small to allow accumulation of fluid. On the other hand, discrete joint effusions can be readily demonstrated with US in the subtalar joint, which has compliant posterior and anterior recesses. Owing to the intrinsic complexity of the foot anatomy, establishing the involved joints and their degree of inflammation can be underestimated by clinical and radiographic evaluation (Smyth and Janson 1997). Involvement of the talonavicular joint and the anterior subtalar joint (possibly associated with sinus tarsi syndrome) is relatively specific for rheumatoid arthritis (Weishaupt et al. 1999). Later in the course of the disease, other midtarsal joints can be involved (Boutry et al. 2005). A recent study performed on a series of patients affected by chronic arthritis has shown that US often re-allocated the site of inflammation to a joint other than that previously reported on the basis of clinical findings and radiographic assessment. A more accurate detection of the exact sites of inflammation has an impact on treatment planning and response to local treatment with steroid injections (D’Agostino et al. 2005). In addition, US offers an accurate real-time guidance for intra-articular steroid injections (Koski 1993, 2000). In advanced disease, laxity of joint capsules and ligaments, tibialis posterior tendon rupture, abnormalities in the sinus tarsi space, and degenerative changes involving the talonavicular and the subtalar joint lead to flatfoot deformity (pes planovalgus).
Neuropathic osteoarthropathy is a complication which mainly occurs in patients affected by diabetes mellitus. This condition usually involves the midtarsal and tarsometatarsal joints and, to a lesser extent, the metatarsophalangeal joints (Ashman et al. 2001). Neuropathic osteoarthropathy seems related to the fact that diabetic patients are more vulnerable to trauma and derangement of joints as a result of impaired sensory and proprioceptive responses. In the acute resorptive phase of the disease, sympathetic nerve dysfunction produces intense edema and hyperemia in the soft tissues of the foot, with joint effusion, bone fragmentation, and progressive destructive changes. Although MR imaging is the modality of choice to examine patients with suspected neuropathic osteoarthropathy, for its ability to depict bone and soft-tissues changes, US is able to identify early irregularities and discontinuity of the cortex of tarsal bones with sensitivity higher than that of plain films. In addition, it can give early depiction of the severe hyperemia and inflammatory response of the soft tissues (Fig. 31d). In many instances, these findings lack sufficient specificity for a definitive diagnosis. This is particularly true when neuropathic osteoarthropathy must be distinguished from osteomyelitis, which represents the main differential diagnosis and may even coexist with it (Marcus et al. 1996). In doubtful cases, biopsy is indicated to exclude infection.
Plantar fasciitis is the most common cause of heel pain (Theodorou et al. 2000, 2001). This condition is a low-grade inflammatory disorder of the fascia that can also involve the perifascial tissues (Dyck et al. 2004; Cole et al. 2005). It is a primary process and should be distinguished from enthesopathy which may occur in seronegative spondyloarthropathy. In most cases, the inflammation results from overuse due to an increased load. Excessive tension applied to the plantar fascia can derive from excessive physical activities (i.e., jumping and running). In specific clinical settings, the excessive chronic load may be related to foot deformities and inadequate biomechanics. A certain correlation with increased body weight has also been described (Kane et al. 2001). Also, in a limited number of patients, plantar fasciitis may be part of systemic disorders, such as rheumatoid arthritis, seronegative spondyloarthropathies, and gout. An increased incidence of plantar fasciitis has been described in patients with Achilles tendon disease (Gibbon and Long 1999).
It is postulated that increased traction of the calcaneal attachment of the plantar fascia leads to local microtears followed by reactive inflammatory changes. In chronic lesions, collagen degeneration and necrosis, angiofibroblastic hyperplasia, and local degenerative changes are found at histopathologic examination. Calcific deposits at the insertion of the abductor hallucis brevis or, less frequently, the abductor digiti minimi following strain injuries are related to enthesopathy and do not represent true calcification of the plantar fascia. Clinically, patients complain of localized pain over the inferomedial aspect of the heel that worsens in the morning and is exacerbated by sporting activities, weight-bearing, and long walks. Often, symptoms persist for months and even years. Physical examination shows a normal heel with exquisite tenderness at the insertion of the fascia on the medial tubercle of the calcaneus. Treatment is conservative and relies on restriction of physical activity, physical therapy with elongation exercises, transcutaneous nerve stimulation, and nonsteroidal anti-inflammatory drugs. In nonresponding patients, local steroid injection, possibly guided by US, is usually effective. The main complications of local steroid administration are rupture of the fascia and hypotrophy of the heel fat pad. In rare instances, surgical fasciotomy is performed.
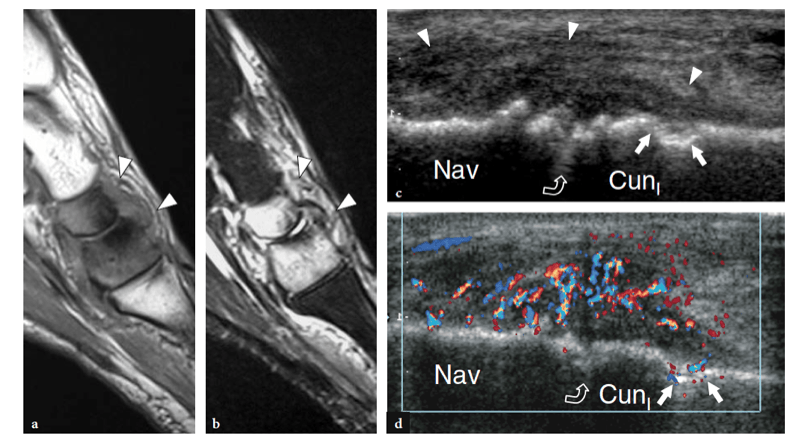
Several articles have described the US appearance of plantar fasciitis (Wall et al. 1993; Cardinal et al. 1996; Gibbon and Long 1999; Akfirat et al. 2003; Sabir et al. 2005). The most common site of pathologic changes is the posterior portion of the fascia, close to its insertion on the medial tubercle (Fig. 32). Although the posterior third of the fascia is selectively affected in most patients, some cases show pathologic abnormalities extending to the middle third (Fig. 33a–d). The cause of this different distribution is unknown. The main US findings include: fascial thickening, hypoechoic echotexture with loss of the fibrillar pattern, blurring of the superficial and deep borders of the fascia and, more rarely, perifascial effusion. As regards fascial thickening, a thickness ≥5 mm indicates fasciitis (Cardinal et al. 1996; Gibbon and Long 1999; Tsai et al. 2000a; Walther et al. 2004). The hypoechoic changes observed in plantar fasciitis are believed to reflect fascial edema resulting from microtears and local degeneration. In 40% of patients affected by acute plantar fasciitis, Doppler imaging may reveal hyperemia in the fascia and the adjacent soft tissues (Walther et al. 2004). The hyperemic pattern is not shown in patients with chronic disease lasting longer than 12 months (Walther et al. 2004).
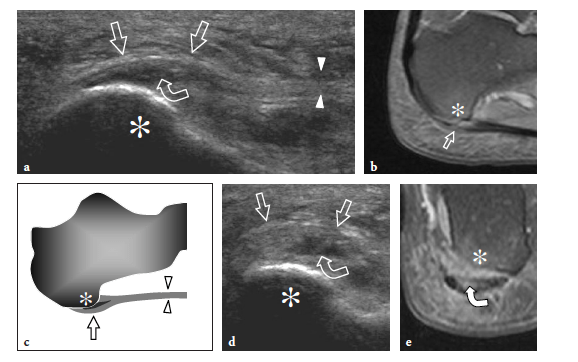
Often, a heel spur is found on the inferior aspect of the calcaneus (Fig. 34). In plantar fasciitis, these spurs seem to be related to a phenomenon reactive to increased tensile forces at the enthesis rather than being the cause of the inflammatory process (Gibbon and Long 1999). US has a definite role in the management of plantar fasciitis by guiding local injection of steroids (Kane et al. 1998; Tsai et al. 2000b, 2006), extracorporeal shock-wave therapy (Hyer et al. 2005b), or needle fasciotomy (Folman et al. 2005). For steroid injection, both posterior and anterior approaches can be used to direct the needle tip inside the thickened portion of the fascia. Similar to other authors, we prefer to select a posterior approach to inject the plantar fascia (Kane et al. 1998; Tsai et al. 2000). The patient lies prone with the affected foot resting on a triangular pillow to obtain knee flexion at approximately 45°. After accurate cleaning of the skin, a 23 gauge needle is inserted through the posterior aspect of the heel.
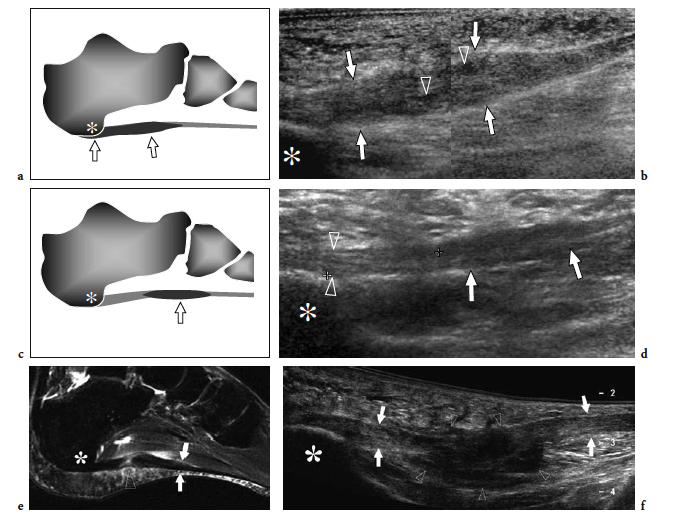
During real-time scanning, the needle is gently advanced parallel to the transducer until its tip reaches the superficial aspect of the fascia. Then, 2–3 ml of 1% lidocaine is slowly injected on the surface of the fascia. One minute later, we perform several “to-and-fro” punctures of the fascia with the needle tip parallel to the fibers. Finally, 1 ml of steroid solution is injected onto the superficial aspect of the fascia (Wong et al. 2002). The procedure is usually well tolerated by the patient. The patient should be advised that, in some cases, a temporary increase in the pain may be noted following the injection. In terms of therapeutic efficacy, US-guided injections seem to be more effective than palpation-guided injections. Compared with the blind technique, US guidance reduces the risk of steroid-induced rupture of the fascia (Sellman 1994). Similarly, fatty atrophy of the plantar heel is less common when the injection is guided by US. After therapy, US shows decreased swelling and a more attenuated hypoechoic pattern of the fascia (Kamel and Kotob 2000; Tsai et al. 2000; Hammer et al. 2005). Some improvement of the US appearance of the fascia has been demonstrated after treatment with extracorporeal shock-wave therapy (Hammer et al. 2005).
Plantar fascia tears are located at the posterior insertion of the fascia. This condition is most often observed in sportsmen who have sustained forceful plantar flexions. The US appearance of plantar fascia rupture is similar to that of plantar fasciitis and the diagnosis relies mainly on clinical and US findings, including focal nodular swelling and a hypoechoic appearance of the fascia (Fig. 33e,f). Surgical fasciotomy exhibits similar characteristics (Yu et al. 1999; Yu 2000). The fascia remains markedly thickened with indistinct superficial and deep margins, probably representing perifascial fibrosis (Fig. 35). US can assess plantar fascia involvement related to inflammatory disorders, such as spondyloarthropathy (Lehtinen et al. 1994; D’Agostino et al. 2003; Borman et al. 2005). Subclinical enthesitis in the feet of these patients is not rare and can easily be detected with US. At gray-scale US and Doppler imaging, the large majority of these patients have at least one sign of active enthesitis that typically presents with symmetric distribution in the extremities.
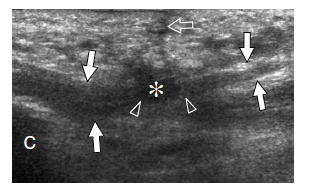
Plantar fibromatosis is a benign condition characterized by focal nodular enlargement of the plantar aponeurosis due to local proliferation of fibrous tissue. Its origin is unknown and it was first described by Dupuytren in 1839, who noted an association with palmar fibromatosis (Dupuytren 1839). This condition is also known as Ledderhose disease after the eponymous doctor who reported more than 50 cases in 1897 (Ledderhose 1897). Patients present with a firm nontender or slightly tender fibrous nodule localized at the medial aspect of the middle third of the sole (Fig. 36). Passive dorsal extension of the toes tightens the aponeurosis and can result in increased local pain. In large lesions, pain may derive from direct compression exerted by the plantar nodule against the medial plantar nerve. Treatment is conservative, based on courses of nonsteroidal antiinflammatory drugs and analgesics. More rarely, surgical excision of the fibrotic nodule is needed. In these cases, complete fasciotomy must be performed to prevent local recurrences.
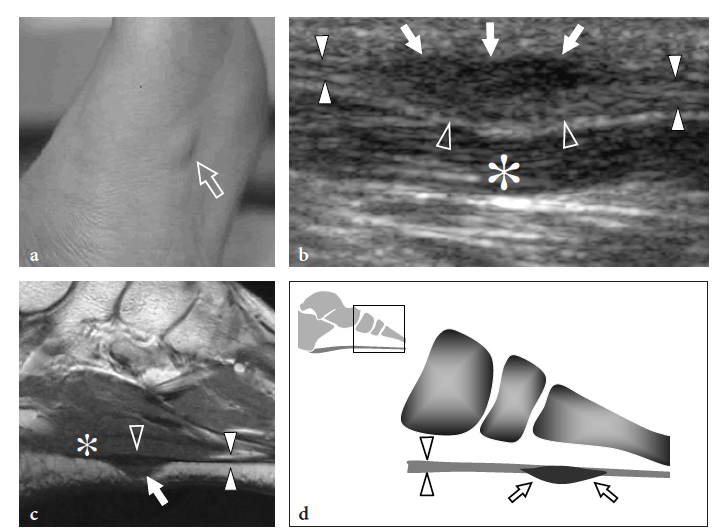
US demonstrates plantar fibromatosis as a fusiform nodular thickening of the plantar fascia oriented according to its major axis. The lesion most often involves the middle third of the plantar fascia and has a uniform hypoechoic appearance without internal cystic or calcific deposits (Griffith et al. 2002). The US appearance of plantar fibromatosis is typical and demonstration of the continuity of the lesion with the fascia excludes other tumors, including synovial sarcoma and soft-tissue fibroma. Some nodules display moderate posterior acoustic enhancement. In small lesions, the deep portion of the fascia is unaffected and exhibits a normal hyperechoic fibrillar structure; in contrast, larger nodules appear more rounded and heterogeneous (Bedi and Davidson 2001). Occasionally, a second smaller nodule can be found in the same or the contralateral foot. Color Doppler imaging can show increased intralesional vasculature. Overall, no correlation has been found among the US appearance of the nodules, the duration of symptoms, and the clinical outcome (Griffith et al. 2002). The main differential diagnosis of plantar fibromatosis is plantar fasciitis. Plantar fasciitis is seen as a thickened and hypoechoic fascia at or near the calcaneal insertion, especially medially, and is often associated with a calcaneal spur. In contrast, plantar fibromatosis gives rise to a plantar mass that is separate from the calcaneus. Distinguishing plantar fibromatosis from a chronic partial tear of the fascia is more difficult and relies on a clinical history of trauma (Reed et al. 1991). Recurrences display a more aggressive pattern with ill-defined borders, mixed echotexture, and a hypervascular pattern: they may require more aggressive treatment (Lee et al. 1993) (Fig. 37).
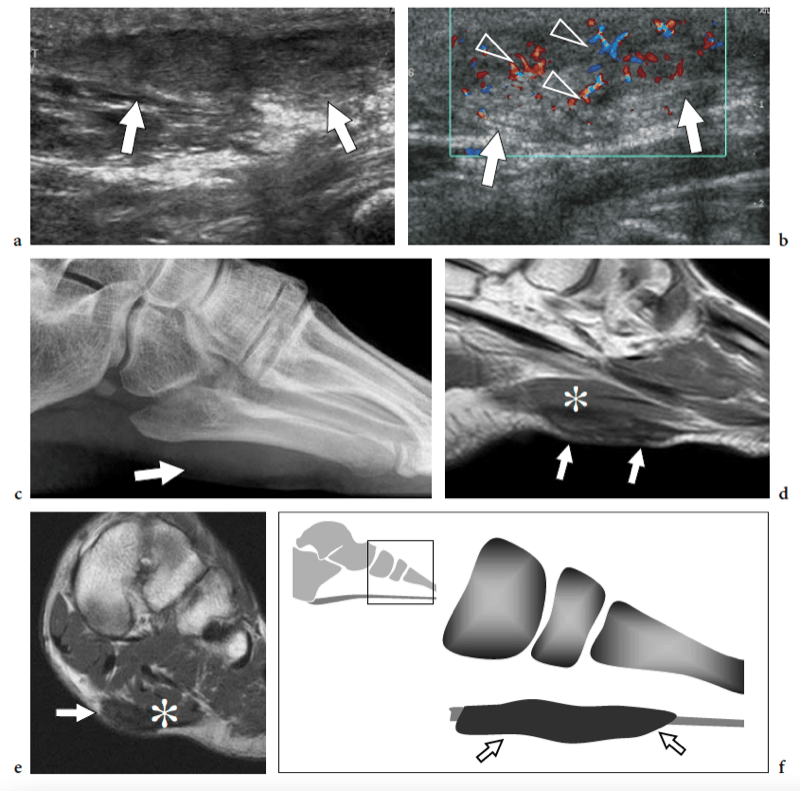
Plantar vein thrombosis is an uncommon condition of unknown origin that may mimic plantar fasciitis (Bernathova et al. 2005). The symptoms include sudden pain in the plantar region with soft-tissue edema of the sole. US demonstrates plantar vein thrombosis as one or more enlarged plantar veins containing hypoechoic noncompressible material, reflecting clots (Fig. 38). Color Doppler imaging may aid the diagnosis. Although MR imaging has been described as a useful tool for the diagnosis of localized thrombosis of the foot veins, US is recommended as the first-line imaging modality (Bernathova et al. 2005). Phlebography is of no use for visualizing plantar vein thrombosis because the intravenous contrast agent is injected proximal to the plantar veins (Bernathova et al. 2005).
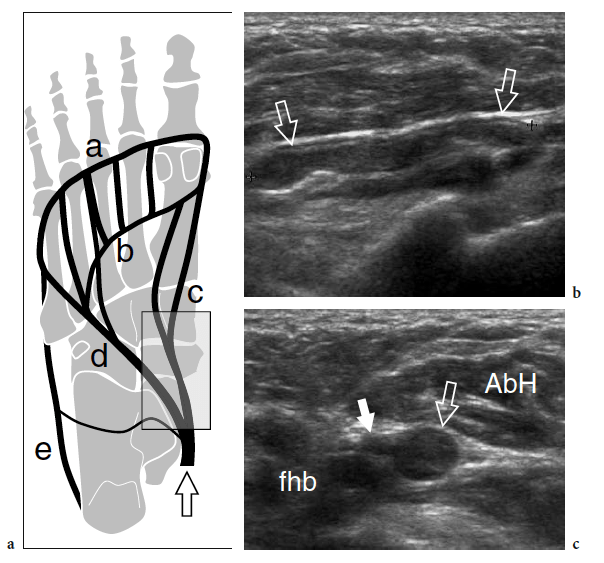
Forefoot pain is a common clinical problem. Several pathologic conditions produce pain in the region of the metatarsal bones and the cause may be difficult to establish based solely on clinical findings. Although radiography is useful in detecting bone lesions, it typically does not help the diagnosis of early bone abnormalities or soft-tissue disease-causing forefoot pain.
Systemic inflammatory diseases affecting the forefoot cover a wide range of pathologic conditions, including rheumatoid arthritis, Reiter’s disease, and psoriasis (Weishaupt et al. 1999). Of these, rheumatoid arthritis affects the forefoot more commonly, and this may be the initial manifestation of the disease in up to 20% of patients. US findings share the characteristics already described for hand arthritis, including joint effusion, thickened synovium, sheath tenosynovitis, bursitis, and erosive changes (Boutry et al. 2005). Generally speaking, a small amount of fluid in the dorsal and plantar recesses of the metatarsophalangeal joints should be regarded as a normal finding (Fig. 39). Similar to other joints, the pannus is demonstrated as a hypoechoic hypertrophy of the synovium that can show hypervascular changes at color and power Doppler imaging in the acute phases of disease (Fig. 40a–c). The examiner should take into account that the US diagnosis of joint effusion requires a larger amount of fluid compared with the contralateral foot and, most importantly, a positive correlation with clinical features. In rheumatoid arthritis, bone erosions appear as irregular defects located in the marginal area: the fifth metatarsophalangeal joint seems the most frequently affected, with erosions involving the lateral aspect of the fifth metatarsal head (Boutry et al. 2005). Therefore, it must be carefully evaluated with US. Intermetatarsal (second and third web spaces) and submetatarsal (first metatarsal head) bursal involvement is commonly associated (Boutry et al. 2005). Signs of tenosynovitis are observed in 48–60% of cases, predominantly affecting the flexor tendon sheath (Boutry et al. 2003; Ostendorf et al. 2004). US-guided intra-articular injection of steroids in the affected synovial spaces is usually less painful than blind injection. The first metatarsophalangeal joint is the most common site of degenerative osteoarthritis in the ankle and foot. Usual radiographic abnormalities include asymmetric joint space narrowing, dorsal and lateral osteophyte formation, subchondral sclerosis, and intra-articular loose bodies. These changes may lead to painful impairment of dorsiflexion of the great toe, so-called hallux rigidus, and are often superimposed on hallux valgus deformity.
Osteoarthritis of the second through the fifth metatarsophalangeal joints is unusual, but it can be seen in any joint which becomes the primary weight-bearing joint (Fig. 40d–f). In the chronic phase of gout arthritis, the most common inflammatory arthritis in adult men, the first metatarsophalangeal is the most frequently involved joint. US can demonstrate monosodium urate crystals in the synovial fluid and the articular and para-articular structures as small hyperechoic foci contained within joint recesses, bursae, and tendon sheaths (Fig. 41). Osteomyelitis of the foot typically occurs in diabetic patients as a result of a contiguous source of infection. In fact, these patients tend to develop ulcers at pressure points (the undersurface of the first and fifth metatarsal heads being the most commonly affected) that may become infected leading to contiguous spread to the underlying metatarsal head and metatarsophalangeal joint (Ashman et al. 2001). For the most part, US findings are nonspecific and may also be encountered in acute neuropathic disease and inflammatory arthritis. Secondary soft-tissue signs of infection, including skin ulcer, cellulitis and soft-tissue abscess, the presence of a sinus tract, and cortical breakage may support the diagnosis of infectious disease.
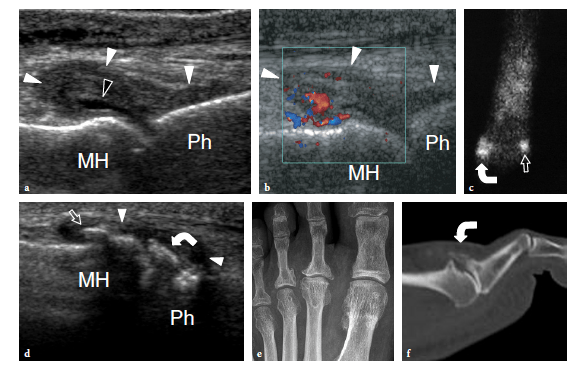
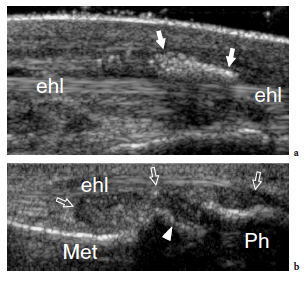
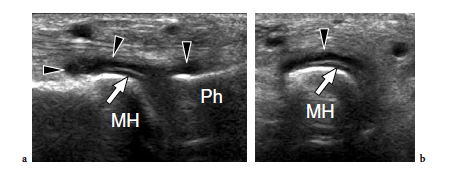
Freiberg disease relates to avascular necrosis of the second metatarsal head that is most often encountered in the second decade, predominantly in women (Freiberg 1926). Patients complain of vague forefoot pain localized over the metatarsophalangeal joint, stiffness, and a limp. Specific signs are swelling and tenderness localized to the metatarsal head. Although this condition can be easily diagnosed on plain films, sonologists must be aware of its US appearance because patients can be submitted to US examination in the absence of previous radiographic studies. Sagittal US images obtained over the dorsal aspect of the second metatarsophalangeal joint are well suited to detecting Freiberg infraction. US can easily show collapse of the metatarsal head with loss of its convexity, secondary degenerative changes, widening of the joint space, and joint effusion (Fig. 43). Intraarticular loose bodies can occasionally be found in the joint recesses in the form of small hyperechoic fragments with posterior acoustic shadowing.
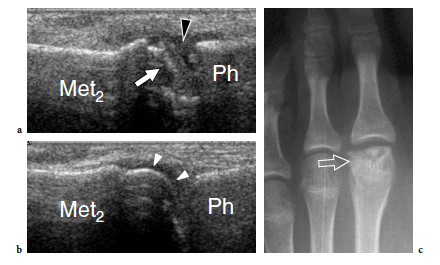
“Insufficiency fractures” occur when a weak bone fails as a result of repetitive loading. This condition typically involve postmenopausal women after prolonged walking. When affecting the foot, insufficiency fractures most commonly involve the shafts of the second and third metatarsals. On the other hand, so-called stress fractures are common in the metatarsal bones of persons whose sporting, recreational, or occupational activities result in repetitive loading of the foot. Runners, dancers, gymnasts, and military recruits after long marches (march fracture) are more vulnerable to metatarsal stress fractures (Weinfeld et al. 1997). Some biomechanical factors, such as a high longitudinal arch, an excessive forefoot varus, and an increased hindfoot inversion, also play a role.
An early diagnosis is difficult because symptoms are often nonspecific and the first standard radiograph performed in the acute phases is usually normal. US is able to detect stress fractures of the metatarsals (Howard et al. 1992; Bodner et al. 2005). When a stress fracture is suspected on clinical grounds, the affected metatarsal is first scanned in the sagittal plane in an attempt to include its full longitudinal extension in a single image. A hypoechoic thickening of the periosteum with an adjacent small fluid collection is often observed (Fig. 44a). Then, short-axis US images may reveal hyperechoic soft-tissue swelling, some fluid, and a hypervascular pattern around the bone (Fig. 44b,c). These signs are highly suggestive of a stress fracture. MR imaging can confirm the diagnosis in the early phase, when radiographs are negative (Fig. 44d,e). In other instances, the fracture may appear as bony cortex irregularities related to callus formation (Fig. 45a). When the callus is mature, the involved bone appears larger in size and characterized by stronger posterior acoustic shadowing compared with the adjacent normal bones (Fig. 45b). The appearance of metatarsal insufficiency fractures is similar, although the abnormalities described above seem to be less pronounced. The periosteal reaction is limited to a smaller segment and soft-tissue edema is less manifest. We believe that, in the proper clinical setting and with negative plain films, the US appearance of insufficiency fractures can be sufficiently specific to suggest conservative treatment and to postpone MR imaging examination. A definitive diagnosis can be made only when repeated radiographs obtained 2–3 weeks after the onset of symptoms demonstrate the callus (Fig. 45c,d). MR imaging should be performed if symptoms do not improve within 1–2 weeks.
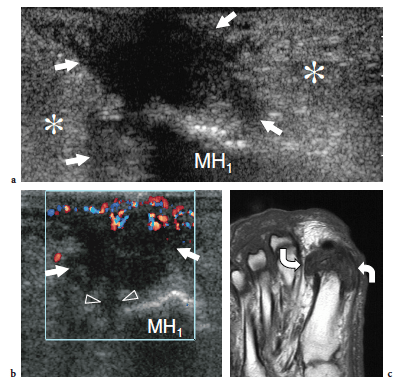
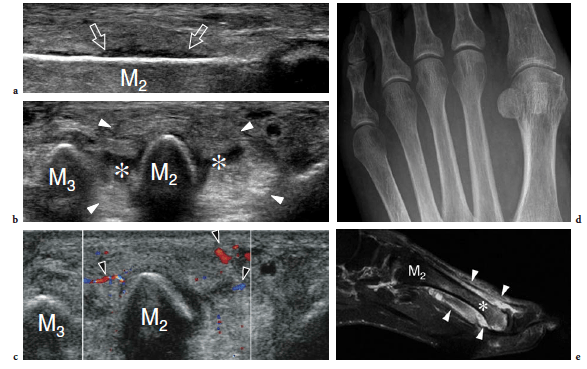
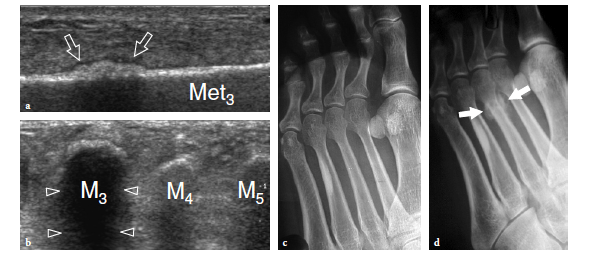
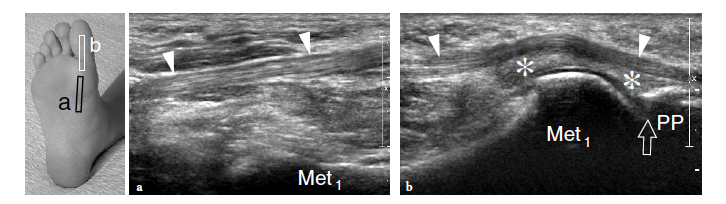
So-called turf toe describes a sprain of the first metatarsophalangeal joint in which there is partial or complete disruption of the plantar plate, presenting with persistent hyperextension of the proximal phalanx. Similar to the volar plate in the hand, the plantar plate is a fibrocartilaginous structure which extends from the plantar aspect of the metatarsal neck to the proximal phalanx. The pathomechanism involves a hyperextension injury at the first metatarsophalangeal joint, possibly resulting from a hard push-off on a rigid surface (Fig. 46). This condition typically occurs in sportsmen (football players) who play on hard, artificial surfaces and wear lightweight flexible shoes (Ashman et al. 2001; Yao et al. 1996). The involvement of the metatarsophalangeal plantar plates of the lesser toes may occur in women with increased weight-bearing load related to high-heeled, pointed shoes (Ashman et al. 2001). Like other fibrocartilaginous structures (e.g., knee meniscus, glenoid labrum), the normal plantar plate appears as a homogeneously hyperechoic structure that reinforces the plantar aspect of the joint capsule. Plantar plate injury manifests either as a swollen hypoechoic and discontinuous structure or with disruption of its attachment to the proximal phalanx. Soft-tissue edema and metatarsophalangeal joint synovitis are associated findings.
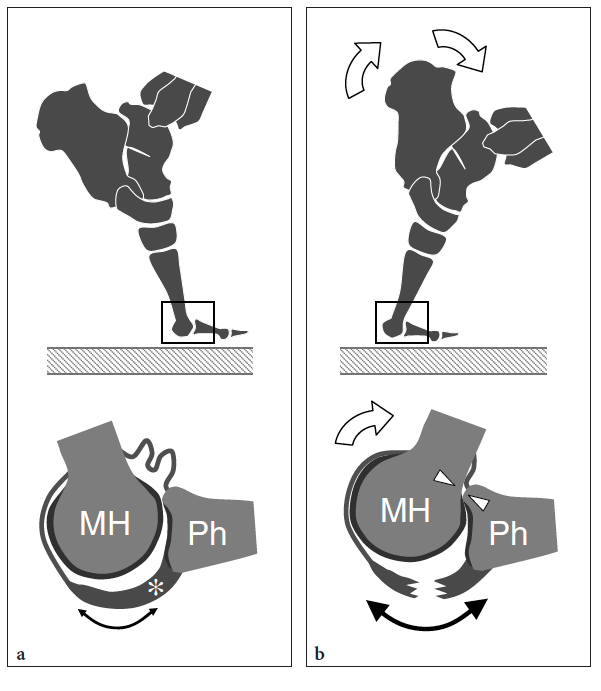
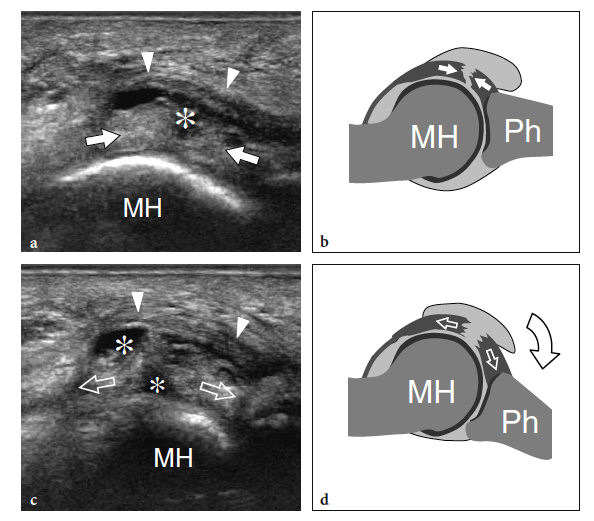
Dynamic scanning during flexion and extension of the affected toe can help the diagnosis by opening the gap of the tear within the substance of the plantar plate (Fig. 47). Passage of joint fluid into the sheath of the adjacent flexor hallucis longus tendon distal to the sesamoids often coexists and cannot be misinterpreted as a simple sign of tenosynovitis (Fig. 48a–c). In doubtful cases, an arthrogram of the affected joint showing opacification of the flexor tendon sheath is pathognomonic for a plantar plate tear (Fig. 48d-g).
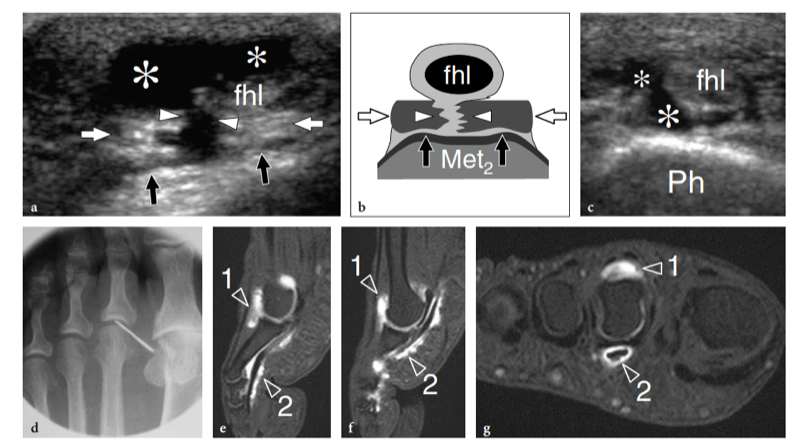
Morton neuroma is a painful condition that mainly occurs in middle-aged women and reflects a mechanically induced degenerative neuropathy of a plantar common digital nerve (Morton 1876; Wu1996, 2000). From the histopathologic point of view, Morton neuroma is not a true neoplasm as it consists of a perineural fibrotic mass associated with vascular proliferation and axonal degeneration. This condition preferentially affects the third and, to a lesser extent, the fourth interdigital nerve as a result of repetitive local trauma: the first and the second web spaces are rarely involved. Some anatomic considerations can explain the more common involvement of the third web space. First, the third common digital nerve is thicker than the other digital nerves as it originates from the union of two branches coming from the medial and lateral plantar nerves. Second, the nerve is located closer to the third metatarsal head and, therefore, is more vulnerable to trauma. Third, the greater mobility between the third and fourth metatarsals can facilitate impingement of the nerve against the intermetatarsal ligament. Finally, at the level of the metatarsal heads, the width of the second and third web spaces is less than that of the first and fourth spaces (Levitsky et al. 1993). Patients with Morton neuroma usually complain of sharp local pain referred between the third and fourth toes with distal irradiation. The pain can be sharp or dull, and is worsened by wearing high-heeled, narrow-toed shoes and by walking. In general, it is less severe when the foot is not bearing weight.
Knowledge of the forefoot anatomy is a prerequisite to understanding the macroscopic and US appearance of Morton neuroma. At the proximal third of the metatarsals, the lateral and medial plantar nerves give off four common plantar digital (interdigital) nerves. These latter nerves proceed straight ahead accompanied by the respective interdigital vessels (artery and veins) to reach the region of the metatarsal heads (Fig. 49a). A cross-sectional view through the web region reveals two spaces: plantar and dorsal (Fig. 49b). The plantar space is delimited as follows: above, by the dorsal intermetatarsal ligament that inserts into the bases of the metatarsal heads reinforced by transverse fibers of the plantar fascia; laterally and medially, by the fibrous sheath of the flexor tendons; below, by the inferior intermetatarsal ligament. The dorsal space is located among the metatarsal heads and houses the interosseous tendons and the synovial intermetatarsal bursa embedded within fat tissue. The intermetatarsal bursa is an attritional bursa that facilitates movement of the structures contained in the dorsal space; in normal subjects, it contains a small amount of fluid. Intermetatarsal fluid is considered abnormal when the bursa has a transverse diameter of ≥3 mm (Zanetti et al. 1997). In the sagittal plane, the common digital nerves course more dorsally as they approach the toes (Fig. 49c). Distal to the deep intermetatarsal ligament, the nerves pierce the inferior intermetatarsal ligament and split in two proper digital nerves that distribute to the contiguous sides of adjacent toes.
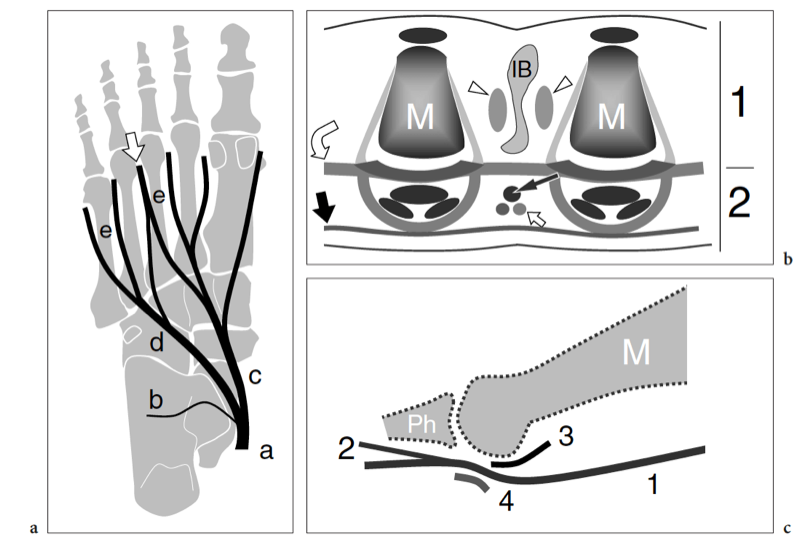
When a Morton neuroma is suspected on clinical grounds, we select a dorsal approach to examine the web spaces because the skin (and especially the stratus corneus of the epidermis) over the dorsum of the foot is thinner than that of the sole and the attenuation of the US beam is less. The patient lies supine or seated on the bed with the knee extended and the ankle in a neutral position. The intermetatarsal spaces should be examined in sagittal planes applying firm pressure with the transducer on the dorsal aspect of the foot while exerting finger pressure in the web spaces from the plantar surface (Fig. 50). The thumb of the hand not holding the probe works well for this purpose. The examiner should invite the patient to relax in order to obtain better displacement of the metatarsal heads and an adequate acoustic window for intermetatarsal assessment. As shown by surgical testing, this maneuver allows the neuroma to sublux around the anterior edge of the intermetatarsal ligament into the dorsal web space (Read et al. 1999). Other authors advocate the use of a plantar approach (probe placed on the plantar foot and the thumb pressed on the dorsal foot), suggesting that the quality of US images obtained over the plantar surface of the foot is higher because the neuroma is closer to the probe (Fig. 51) (Oliver and Beggs 1998; Quinn et al 2000). However, there is no convincing evidence that the plantar approach is superior to the dorsal one. Whatever the selected approach (dorsal or plantar), all intermetatarsal spaces must be carefully explored because Morton neuromas may be multiple. If findings suggestive of Morton neuroma are identified, the examiner must apply further compression over the affected web space with the following aims: to displace any fluid related to coexisting intermetatarsal bursitis (to allow precise measurement of the neuroma and diagnosis of bursitis); to confirm the causative role of the mass in the generation of pain (sonographic Tinel sign). This latter sign becomes more manifest if the examiner squeezes the metatarsal heads with the hand not holding the probe. To further increase diagnostic confidence and overall accuracy, pressure can be applied on the medial and lateral aspects of the forefoot while relieving pressure with the transducer on the plantar foot in an attempt to demonstrate the neuroma, squeezed between the metatarsal heads, as it abruptly displaces toward the plantar surface of the foot causing a palpable click, the so-called sonographic Mulder sign (Fig. 52) (Torriani and Kattapuram 2003).
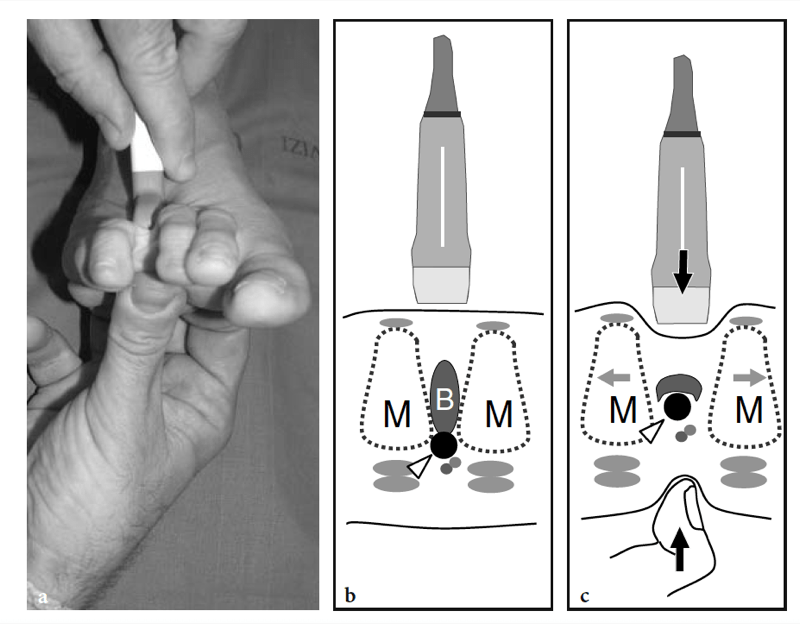
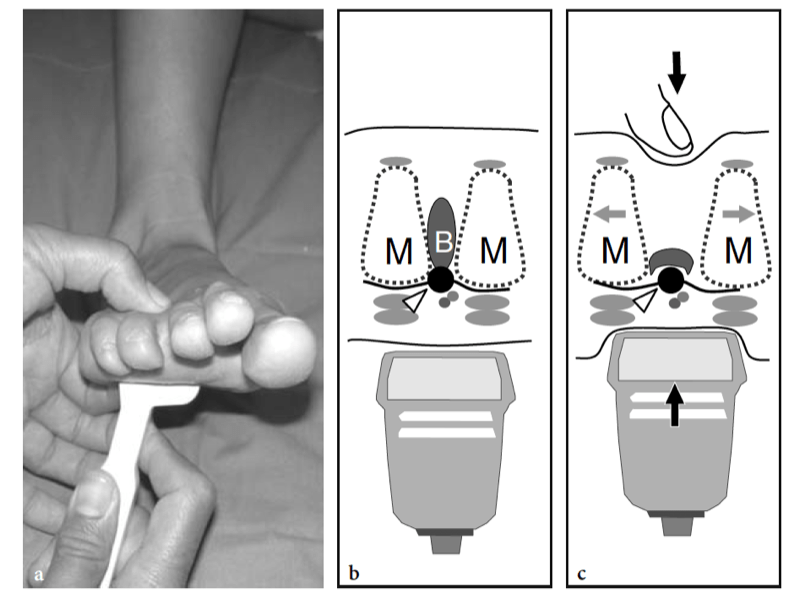
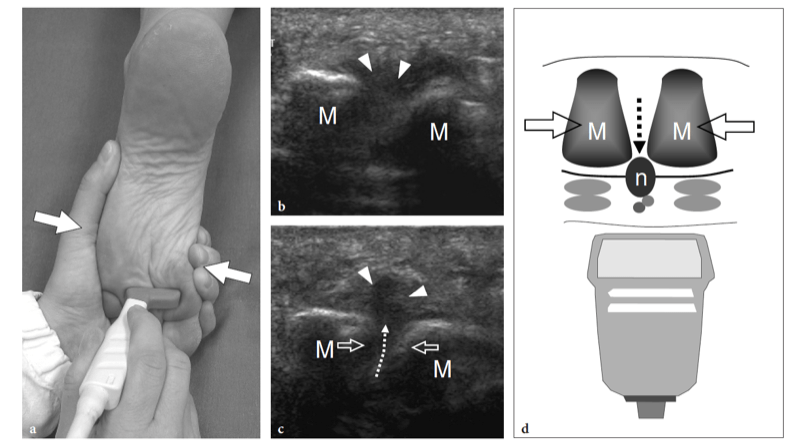
The US appearance of Morton neuromas depends on the selected scanning plane. On longitudinal US images obtained from a dorsal approach, Morton neuroma appears as a fusiform hypoechoic mass with its long axis oriented obliquely to the metatarsals (Fig. 53a). Transverse US planes depict Morton neuromas as hypoechoic rounded lesions that have a smaller size than in the sagittal plane and are surrounded by hyperechoic tissue (Fig. 53b–e). The internal echotexture of Morton neuroma may be hypoechoic, anechoic, or mixed (Quinn et al. 2000). At least in part, mixed-type neuromas seem to be related to coexisting intermetatarsal bursitis (Fig. 54). Some authors have noted that the size of neuroma as measured with US is larger than that found at surgical exploration. This can be explained by the fact that sonologists include in the measurement of neuroma the associated distended intermetatarsal bursa and even some mucoid degeneration of adjacent tissues due to their similar hypoechoic pattern. When distended by fluid, the intermetatarsal bursa appears as an echo-free structure with posterior acoustic enhancement that lies dorsal and posterior to the neuroma (Fig. 54). Compression can displace the bursal fluid and may cause a slight decrease in size of the neuroma as a result of concomitant compression of the adjacent area of mucoid degeneration (Fig. 55). The oblique orientation of the neuroma and the assessment of its continuity with the common digital nerve improve the diagnostic confidence (Fig. 54) (Quinn et al. 2000). Overall, US has proved to be an accurate means of detecting Morton neuroma, with a 100% sensitivity and 83.3% specificity (Shapiro and Shapiro 1995; Sobiesk et al. 1997). Morton neuromas are symptomatic if they measure >5 mm in size when examined along their short axis (Redd et al. 1989; Pollak et al. 1992; Zanetti et al. 1997). In the rare instances in which a restricted web space prevents an accurate US examination, MR imaging should be performed to detect neuromas and distinguish them from other local disorders of the forefoot (Zanetti and Weishaupt 2005). As detailed in Chapter 18, US can successfully guide steroid or alcohol injections to treat symptomatic Morton neuromas (Dockery 1999; Fanucci et al. 2004). In the postsurgical setting, US has proved useful in detecting recurrences (Levine et al. 1998). However, the interpretation of postsurgical findings is often difficult with US because of local scar tissue.
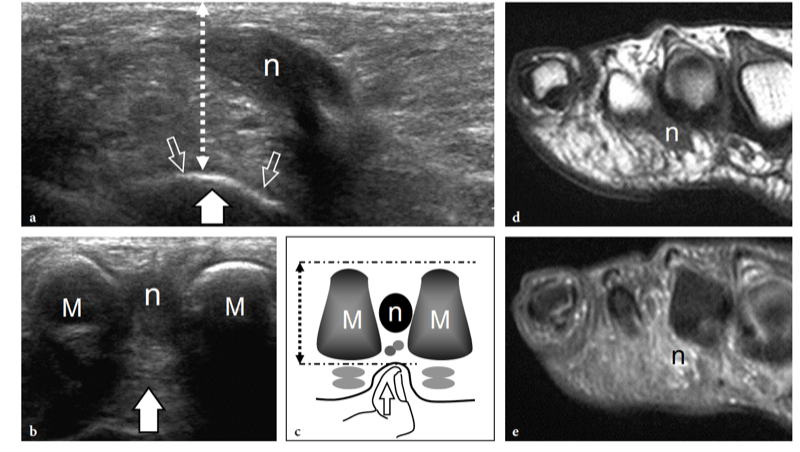
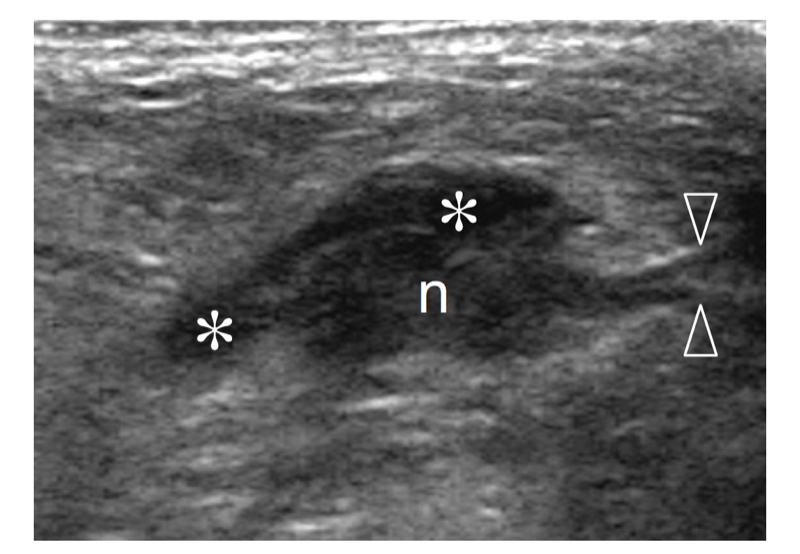
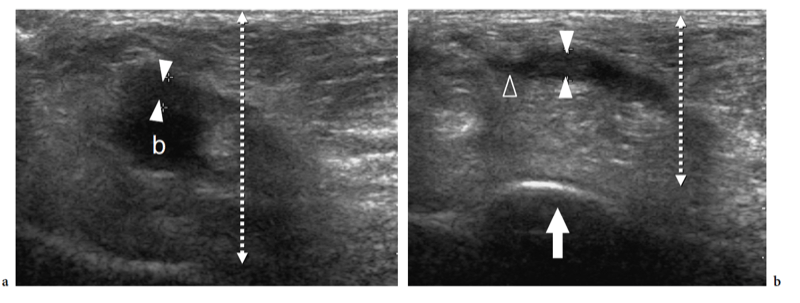
Most soft-tissue masses of the foot are benign nonneoplastic conditions, including ganglion cysts, bursitis, foreign body granuloma, plantar fibromatosis, pigmented villonodular synovitis, and giant cell tumor of the tendon sheath. The foot is the third most common location of ganglion cysts following the wrist and hand (Waldt et al. 2003). In the foot, ganglia most often develop from the tarsal sinus and the tarsal canal (34%), around the Lisfranc joint (14%), and dorsal to the metatarsophalangeal joints (Ashman et al. 2001; Woertler et al. 2005; Weishaupt et al. 2001). Dorsal ganglia usually arise from a tendon sheath, are palpable, and are larger than those located in the hand and wrist (Waldt et al. 2003). Most are clinically asymptomatic (Woertler et al. 2005; Weishaupt et al. 2001). The US appearance of ganglion cysts is variable, ranging from round to oval and lobulated masses (Fig. 56).
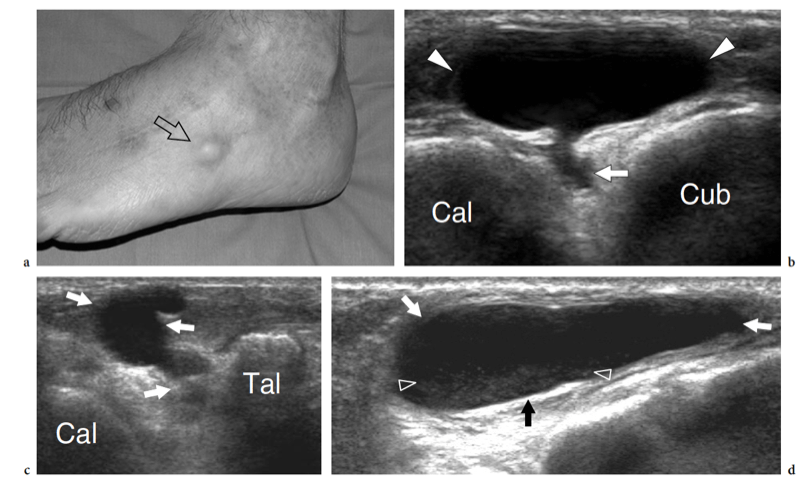
Apart from intermetatarsal bursitis, superficial palpable bursitis can occur in the midfoot over a hypertrophied peroneal tubercle and in the forefoot involving the adventitial bursae beneath the metatarsal heads (Ashman et al. 2001). A distended bursa underlying the head of the first metatarsal is often observed in association with hallux valgus (Schweitzer et al. 1999). It can be recognized as a focal mass with mixed echotexture (containing fluid and hypertrophied synovium) interrupting the subcutaneous fat plane focally (Fig. 57).
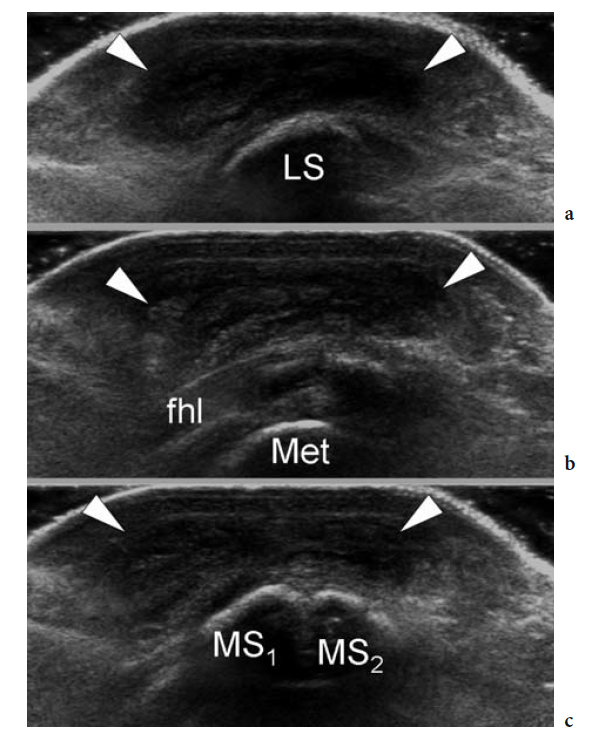
Foreign body granulomas develop in response to fragments of wood, thorns, glass or plastic objects that have penetrated the soft tissues of the foot. They are almost invariably found in the subcutaneous fat at the plantar aspect of the foot, particularly in subjects who walk barefoot. (Fig. 58). The intra-articular form of pigmented villonodular synovitis is a monoarticular condition that can arise as a single nodule or a diffuse villonodular mass, most often located in the ankle and hindfoot (Fig. 59a,b) (Yang et al. 1998; Woertler et al. 2005). In the forefoot, giant cell tumor of the tendon sheath shows a predilection for a location among the toes (Fig. 59c–f). In this area, it represents by far the most common solid benign soft-tissue mass (Ashman et al. 2001). US demonstrates giant cell tumor as a painless solid hypoechoic nodule with a hypervascular pattern located adjacent to or enveloping a tendon (Fig. 59c). Especially in lesions with marked hemosiderin content, low T2-signal intensity areas are typical at MR imaging (Fig. 59d–f).
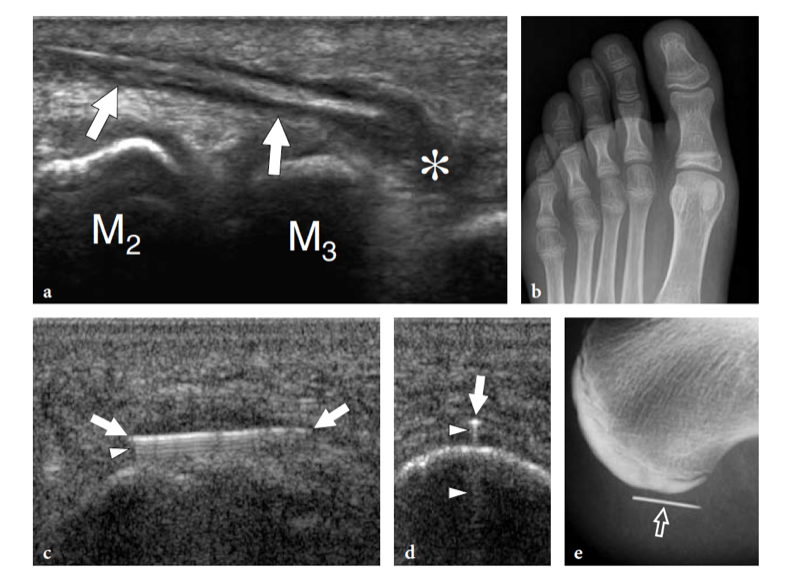
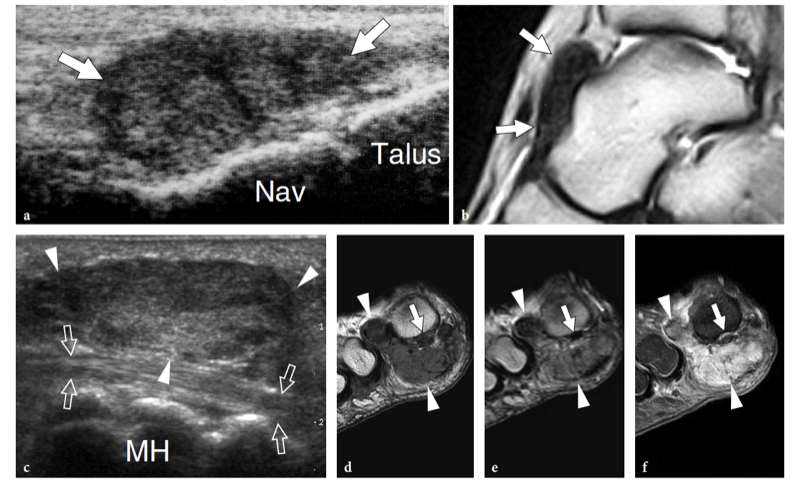
Among primary malignant soft-tissue neoplasm of the foot, synovial sarcoma and the clear cell sarcoma should be mentioned. Synovial sarcoma is the most common primary malignant tumor of the foot and ankle. It rarely arises in an intra-articular location because this histotype has no relationship with synovial tissue (Waldt et al. 2003). The neoplasm is prevalent in adolescent and young adults and develops in the juxta-articular regions of the foot (adjacent to tendon sheath, bursae, ligaments, etc.) as a deep-seated mass associated with insidious pain (Woertler et al. 2005). Large masses contain solid, cystic, hemorrhagic components and, therefore, appear markedly heterogeneous at US, with mixed echotexture (Fig. 60).
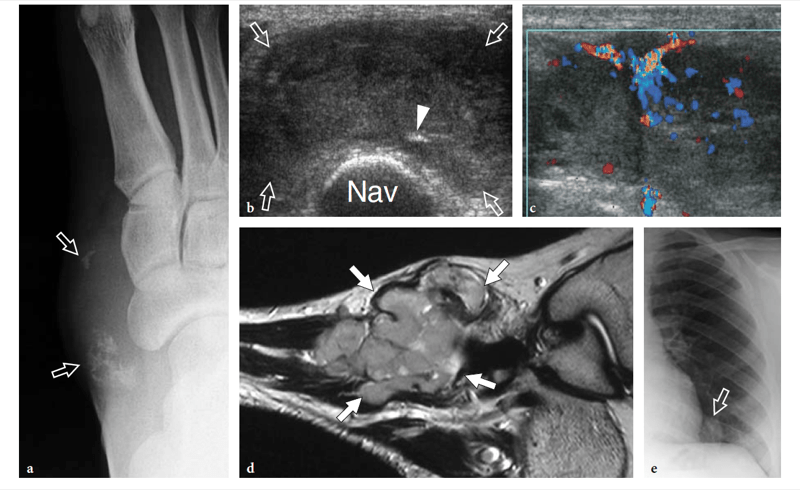
Clear cell sarcoma (malignant melanoma of soft parts) is a highly malignant sarcoma of melanocytic differentiation that occurs in young and middle-aged adults. The tumor is a slowly growing mass characterized by a heterogeneous appearance, well-defined borders (which may mislead the sonologist to diagnose a benign lesion), and a hypervascular pattern at color Doppler imaging; it may cause erosion of adjacent bone (Fig. 61a,b) (Waldt et al. 2003; Woertler et al. 2005). The paramagnetic effect of melanin can cause shortening of T1 and T2 relaxation times at MR imaging examination. Clear cell sarcoma usually shows diffuse strong gadolinium enhancement (Fig. 61c). Both synovial and clear cell sarcoma have nonspecific findings at US. Diagnostic errors can be avoided, however, if any mass of the soft tissues of the foot that does not reveal typical features of a given benign condition is regarded as potentially malignant until proved otherwise.
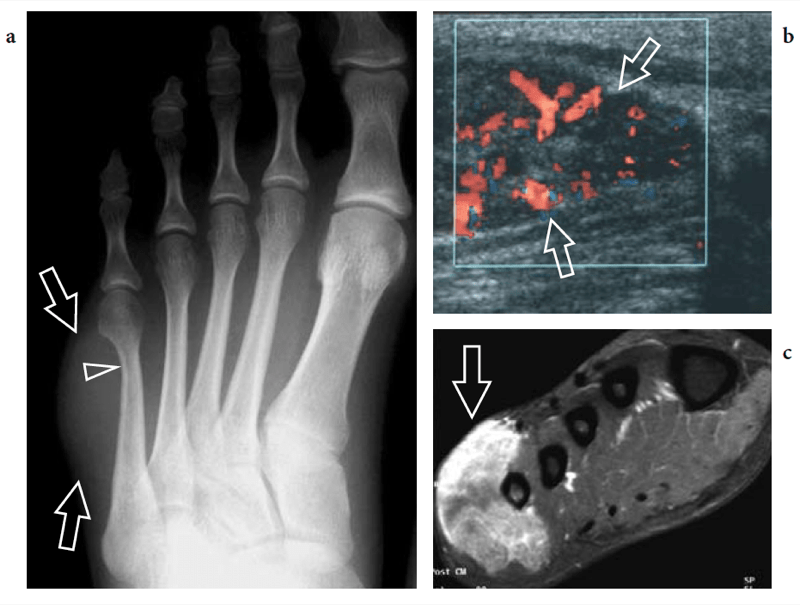
Authors: Stefano Bianchi and Carlo Martinoli