
The discovery of inhaled anesthesia reflects the contributions of clinicians and scientists in the United States and England (Fig. 1) (1). The most commonly used inhaled anesthetics in modern anesthesia include volatile liquids (i.e., halothane, enflurane, isoflurane, desflurane, and sevoflurane) and a single gas (i.e., nitrous oxide) (Figs. 2 and 3). Halothane, enflurane, and isoflurane are no longer commonly used. Yet, none of these inhaled anesthetics meets all the criteria of an “ideal” inhaled anesthetic, and the chemical characteristics differ among the drugs (Table 1).



| Table 1. Comparative Characteristics of Inhaled Anesthetics. | ||||||
|---|---|---|---|---|---|---|
| Characteristic | Isoflurane | Enflurane | Halothane | Desflurane | Sevoflurane | Nitrous Oxide |
| Partition coefficient | ||||||
| Blood-gas Brain-blood Muscle-blood Fat-blood |
1.46 | 1.9 | 2.54 | 0.45 | 0.65 | 0.46 |
| 1.6 | 1.5 | 1.9 | 1.3 | 1.7 | 1.1 | |
| 2.9 | 1.7 | 3.4 | 2.0 | 3.1 | 1.2 | |
| 45 | 36 | 51 | 27 | 48 | 2.3 | |
| MAC (age 30-55 years) % of 1 atmosphere | 1.15 | 1.63 | 0.76 | 6.0 | 1.85 | 104 |
| Vapor pressure at 20° C (mm Hg) | 240 | 172 | 244 | 669 | 160 | |
| Molecular weight (g) | 184.5 | 184.5 | 197.4 | 168 | 200 | 44 |
| Stable in hydrated CO2 absorbent | Yes | Yes | No (a) | Yes | No (a) | Yes |
| Stable in dehydrated CO2 absorbent | No | No (ab) | No (b) | No (abc) | Yes | |
| Percent metabolized | 0-0.2 | 15-40 | 0-0.2 | 5-8 | ||
(a) Compound A.
(b) Carbon monoxide.
(c) Severe exothermic reactions reported.
MAC, Minimum alveolar concentration.
Nitrous oxide gas was first synthesized in 1772 by the English chemist, author, and Unitarian minister Joseph Priestley. Twenty-seven years later, Sir Humphry Davy administered nitrous oxide for dental analgesia. Although he suspected that nitrous oxide might be used to relieve pain for surgery, it was not until it was used 42 years later by a 29-year-old dentist named Horace Wells who administered nitrous oxide to himself and found that it relieved his pain. Specifically, he noticed the hypnotic and analgesic effects of nitrous oxide at a public exhibition in Hartford, Connecticut, in 1842. The next day, Wells himself underwent a dental extraction by a fellow dentist. Wells felt only minimal pain with the extraction, and he subsequently learned the method of nitrous oxide synthesis to make it available to his own patients. Two years later, he arranged to demonstrate painless dental surgery using nitrous oxide administration at the Massachusetts General Hospital. Not being completely successful, Wells was discredited as a result of this demonstration.
William Morton, a Boston dentist, noticed that diethyl ether, during “ether frolics” in which ether was breathed for its inebriating effects, had similar effects as nitrous oxide. Like Wells, Morton applied ether in his dental practice and then demonstrated its anesthetic properties at the Massachusetts General Hospital on October 16, 1846 (“ether day”). In contrast to Wells’s debacle, Morton's demonstration was received with great enthusiasm. The results of successful ether anesthetics were soon published in the Boston Medical and Surgical Journal. Although Crawford Long administered diethyl ether to a patient in 1842, 4 years earlier than Morton, he did not publicize his work, and Morton, therefore, has traditionally been credited with the discovery of diethyl ether’s capability to produce anesthesia.
James Simpson, an obstetrician from Edinburgh, Scotland, developed chloroform, which did not share the protracted induction, flammability, and postoperative nausea seen with diethyl ether. Chloroform soon became popular as an inhaled anesthetic in England, although diethyl ether dominated medical practice in North America. Unfortunately, chloroform was associated with several unexplained intraoperative deaths of otherwise healthy patients and numerous cases of hepatotoxicity.
Between 1920 and 1940, ethylene, cyclopropane, and divinyl ether were introduced into use as anesthetics, gaining acceptance over the older inhaled anesthetics (with the exception of nitrous oxide) by producing a faster, more pleasant induction of anesthesia and by allowing faster awakening at the conclusion of surgery. However, each had serious drawbacks. Many were flammable (i.e., diethyl ether, divinyl ether, ethylene, and cyclopropane), whereas others, halogenated entirely with chlorine, were toxic (i.e., chloroform, ethyl chloride, and trichloroethylene).
Techniques of fluorine chemistry, developed from efforts to produce the first atomic weapons, found a fortuitous, socially beneficial purpose in providing a method of synthesizing modern inhaled anesthetics (2,3). Modern inhaled anesthetics are halogenated partly or entirely with fluorine (see Fig. 2). Fluorination provides greater stability and lesser toxicity.
Halothane was introduced into clinical practice in 1956 and became widely used. It had several advantages compared with the older anesthetics, including non-flammability, a pleasant odor, lesser organ toxicity, and pharmacokinetic properties allowing a much faster induction of anesthesia and emergence compared with ether. Unfortunately, after 4 years of commercial use, reports of fulminant hepatic necrosis after halothane anesthesia began to appear in patients in which other causes of liver damage were not evident. The issue of unpredictable liver damage stimulated the search for other volatile anesthetics. Halothane also sensitizes the myocardium to the dysrhythmogenic effects of catecholamines.
Methoxyflurane was first introduced into clinical practice in 1960. Within the first decade of its introduction, reports of renal failure with methoxyflurane anesthesia appeared, leading to studies confirming a dose-related nephrotoxicity because of the inorganic fluoride that resulted from the metabolism of this anesthetic.
Enflurane was introduced to clinical practice in 1972. Unlike halothane, it did not sensitize the heart to catecholamines and was not associated with hepatotoxicity. However, enflurane was metabolized to inorganic fluoride and could cause evidence of seizure activity on the electroencephalogram (EEG), especially when administered at high concentrations and in the presence of hypocapnia.
Isoflurane was introduced into clinical practice in 1980 and was widely used clinically. It was not associated with cardiac dysrhythmias. Because it is not metabolized as readily as halothane and enflurane, isoflurane was associated with less toxicity. Isoflurane allowed a more rapid onset of surgical anesthesia and faster awakening compared with its predecessors.
Sevoflurane and desflurane are halogenated exclusively with fluorine and were first synthesized during the late 1960s and 1970s, respectively (2,3). Both were expensive and difficult to synthesize and were therefore not immediately considered for commercial use. In the 1980s, their development was reconsidered in light of a new appreciation that a growing proportion of anesthetic practice was taking place in the outpatient setting and that drugs halogenated exclusively with fluorine were less soluble in blood and tissues, allowing faster awakening and recovery (Box 1).
| Box 1. Factors That Increase or Decrease Anesthetic Requirements. |
|---|
|
Factors Increasing MAC |
|
Factors Decreasing MAC |
MAC, Minimum alveolar concentration.
The question of how inhaled anesthetics produce the anesthetic state may be addressed at many levels of biologic organization, including their location of action within the central nervous system, the molecules with which they interact, and the nature of this biologic interaction. Answering these questions requires an ability to measure anesthetic effects (4). Although inhaled anesthetics have been used to provide surgical anesthesia for almost 160 years, there is no single, accepted definition of what constitutes the anesthetic state. For experimental purposes, an operational definition of immobility in response to surgical stimulation and amnesia for intraoperative events has proved useful.
Measurable and universal characteristics of all inhaled anesthetics include production of immobility and amnestic effects. Immobility is measured by the minimum alveolar concentration (MAC) of anesthetic required to suppress movement to a surgical incision in 50% of patients (2,5). However, the presence of amnesia or awareness is difficult to assure. Although analgesia is part of the anesthetic state, it also cannot be measured in an immobile patient who cannot remember. Surrogate measures of pain (i.e., increased heart rate or systemic arterial blood pressure) suggest that inhaled anesthetics do not suppress the perception of painful stimuli. Some inhaled anesthetics have hyperalgesic (pain-enhancing) effects in small concentrations. Skeletal muscle relaxation is a common, but not universal, central effect of inhaled anesthetics, as evidenced by nitrous oxide, which increases skeletal muscle tone.
Immobility
Potent inhaled anesthetics produce immobility mostly by their actions on the spinal cord, as evidenced by determination of MAC in decerebrate animals (6). Studies in rodents suggest that nitrous oxide activates descending noradrenergic pathways originating in the periaqueductal gray matter brainstem, which in turn inhibit nociceptive input in the dorsal horn of the spinal cord (7,8).
Amnestic Effects
Supraspinal structures such as the amygdala, hippocampus, and cortex are considered highly probable targets for the amnestic effects of anesthetics.
Central Nervous System Depression and Ion Channels
Inhaled anesthetics produce central nervous system depression by their actions on ion channels, which govern the electrical behavior of the nervous system (4). Inhaled anesthetics probably produce anesthesia by enhancing the function of inhibitory ion channels and by blocking the function of excitatory ion channels. Enhancing the function of inhibitory ion channels leads to hyperpolarization of the neuron. Hyperpolarization results when chloride anions enter neurons through γ-aminobutyric acid A (GABAA) receptors or glycine receptors or when there is an efflux of potassium cations out of neurons through potassium ion channels. Blocking the function of excitatory ion channels prevents depolarization of the neuron by preventing the passage of positive charges into the neuron (i.e., passage of sodium ions through N-methyl-D-aspartate [NMDA] receptors or sodium channels). Anesthetics may also affect the release of neurotransmitters, and this effect may be mediated in part by ion channels that regulate the release of neurotransmitters.
Modern inhaled anesthetics, with the exception of nitrous oxide, are halogenated hydrocarbons. Halothane lacks the ether moiety present on isoflurane, sevoflurane, and desflurane, accounting for its capability to produce ventricular cardiac dysrhythmias. Isoflurane and desflurane differ only by the substitution of one chlorine atom for fluorine. Fluorine substitution confers greater stability and resistance to metabolism.
Nitrous oxide exists as a gas at ambient temperature, although it becomes a liquid at higher pressures. The remaining inhaled anesthetics are liquids at ambient temperatures.
Variable-Bypass Vaporizers
Halothane, sevoflurane, and isoflurane are delivered by variable-bypass vaporizers (Tec 4, 5, and 7; North American Draeger 19.n and 20.n). The variable-bypass vaporizer contains two streams of inflowing fresh gas—one contacting a reservoir (sump) of liquid anesthetic and the other bypassing the sump. The stream of gas traversing the sump becomes saturated with anesthetic as governed by the anesthetic’s saturated vapor pressure. Because the volatile anesthetics produce clinically useful anesthesia at partial pressures far below that of their saturated vapor pressure, the gas exiting the sump must be diluted by gas that has not come into contact with anesthetic. The concentration of anesthetic in the gas exiting the vaporizer is determined by the relative flow (i.e., splitting ratio) of fresh gas through the sump channel versus the bypass channel. Control of anesthetic output concentration exiting the vaporizer occurs when the clinician adjusts the vaporizer dial or electronic control. Variable-bypass vaporizers are temperature compensated, maintaining constant output over a wide range of temperatures, and are calibrated for each individual anesthetic according to its differing vapor pressure. Tilting or overfilling of a vaporizer can potentially lead to delivery of an overdose of anesthetic if anesthetic vapor gets into the bypass channel.
The Datex-Ohmeda Aladin Cassette Vaporizer, used in the Datex-Ohmeda Anesthesia Delivery Unit (ADU) machines, is a single electronically controlled vaporizer with its bypass housed within the ADU and the sump located within interchangeable, magnetically coded cassettes for delivery of halothane, enflurane, isoflurane, sevoflurane, and desflurane. The Aladin utilizes variable bypass as a means of regulating output concentration, doing so via activity of a central processing unit (CPU). The CPU receives input from multiple sources, including the concentration setting, flowmeters, and internal pressure and temperature sensors, and in turn regulates a flow control valve at the outlet of the vaporizing chamber. If pressure in the cassette (sump) exceeds that in the bypass chamber, which would occur if room temperature exceeds 22.8° C during desflurane administration, a one-way check valve is designed to close, preventing retrograde flow of anesthetic saturated gas back into the ADU with subsequent anesthetic overdose.
Heated Vaporizer
The vapor pressure of desflurane at sea level is 700 mm Hg at 20° C (near boiling state at room temperature), and delivery by a variable-bypass vaporizer can produce unpredictable concentrations. For this reason, a specially designed vaporizer (Tec 6, Datex-Ohmeda) that heats desflurane gas to 2 atm of pressure is used to accurately meter and deliver desflurane vapor corresponding to adjustments of the concentration dial by the anesthesia provider. In contrast to the variable-bypass vaporizers, the output concentration of desflurane from the Tec 6 is constant across a range of barometric pressures (9). Therefore, at high altitudes, the partial pressure of desflurane will be lower at a given Tec 6 vaporizer setting and output (volume percent) concentration than at sea level, leading to underdosing of the anesthetic unless an adjustment is made accounting for the higher altitude: required vaporizer setting = (desired vaporizer setting at sea level × 760 mm Hg)/local barometric pressure (in mm Hg) (10). The converse (a greater output) can occur with variable-bypass vaporizers. However, the pharmacologically relevant quantitative parameter for anesthetic activity is partial pressure, not volume percent. Therefore, although a larger output of anesthesia from a vaporizer occurs at higher altitude for the same vaporizer setting, the delivered partial pressure, and anesthetic impact, will be similar in both locations as related to the vaporizer setting.
Fresh gas flow rate directly impacts the quantity of volatile liquid used, and consequently the cost of the anesthetic delivery. Higher fresh gas flows (at or above minute ventilation) minimize rebreathing and allow faster equilibration between inspired and central nervous system (CNS) partial pressures. However, use of nonrebreathing flows involve loss of anesthetic to the environment and should be used only for a limited period of minutes, usually at anesthetic induction, or in the circumstance of light sedation and imminent surgical stimulation. There is growing awareness and concern regarding the contribution of inhaled anesthetic release to overall greenhouse gas emission and climate change. Potential environmental impact appears to stem from the atmospheric lifetime gas, as well as unique infrared absorption spectrum, of each anesthetic. Atmospheric longevities of inhaled anesthetics differ substantially (nitrous oxide, desflurane, sevoflurane, and isoflurane having 114, 10, 3.6, and 1.2 estimated years, respectively). Individual infrared absorption spectra differ, with desflurane relatively possessing the greatest carbon dioxide equivalent impact when compared to sevoflurane with the lowest. Although impact of inhaled anesthetics to overall climate change remains a topic of controversy, several points warrant consideration. First, use of low fresh gas flows (0.5-1 L/min) will offset cost and release to the environment. Second, development of systems to reclaim and reuse anesthetics hold promise to further limit environmental impact and save money (11).
Anesthetic degradation by metabolism or by an interaction with carbon dioxide absorbents (especially when desiccated) produces several potentially toxic compounds (11).
Metabolism and Degradation
Methoxyflurane produces inorganic fluoride, which was responsible for the sporadic incidence of nephrotoxicity (i.e., high-output renal failure) after prolonged anesthesia in the past. Compound A (i.e., fluoromethyl-2,2-difluoro-1-[trifluoromethyl] vinyl ether), produced from the breakdown of sevoflurane, and a similar compound produced from halothane are nephrotoxic in animals after prolonged exposure. In humans, prolonged anesthesia with sevoflurane and low fresh gas flows (1 L/min) results in compound A exposure adequate to produce transient proteinuria, enzymuria, and glycosuria, but there is no evidence of increased serum creatinine concentrations or long-term deleterious effects on renal function. Nevertheless, the package insert for sevoflurane recommends low fresh gas flow (<2 L/min) be restricted to less than 2 MAC hours (i.e., MAC concentration × duration of administration) of sevoflurane anesthesia.
Carbon Dioxide Absorbents and Exothermic Reactions
Variables influencing the amount of volatile anesthetic degradation on exposure to carbon dioxide absorbents include the condition (i.e., hydration and temperature) and chemical makeup of the absorbent, fresh gas flow rates, minute ventilation, and, most important, the anesthetic itself (12). Although desflurane and isoflurane are very stable in hydrated carbon dioxide absorbents up to temperatures of more than 60° C, full desiccation of conventional carbon dioxide absorbents containing sodium and potassium hydroxide causes degradation and carbon monoxide production from all volatile anesthetics regardless of temperatures. High fresh gas flow rates (especially those exceeding normal minute ventilation) accelerate the desiccation of absorbent, and the desiccation leads to accelerated degradation. Because degradation is an exothermic process, the absorbent temperature may increase dramatically.
The exothermic reaction that results from interaction of desiccated carbon dioxide absorbent and volatile anesthetics (especially sevoflurane) can produce extremely high temperatures inside the absorbent canister (13,14). The temperature increase may lead to explosion and fire in the canister or anesthetic circuit. The remote risk of fire and explosion from exothermic reactions can be avoided entirely by employing measures that ensure maintenance of adequate hydration in the carbon dioxide absorbent (i.e., changing the absorbent regularly, turning fresh gas flow down or off on unattended anesthesia machines, limiting fresh gas flow rates during anesthesia, and when in doubt about the hydration of the absorbent, changing it). Commercially available carbon dioxide absorbents with decreased or absent monovalent bases (i.e., sodium hydroxide and potassium hydroxide) do not undergo extensive degradation on exposure to volatile anesthetics, regardless of the absorbent hydration status.
The relative potency between inhaled anesthetics is most commonly described by the dose required to suppress movement in 50% of patients in response to surgical incision (5). This dose (a single point on a dose-response curve) is designated the MAC. Because the standard deviation in the MAC is approximately 10%, 95% of patients should not move in response to incision at 1.2 MAC of the inhaled anesthetic, and 99% of patients should not move in response to incision at 1.3 MAC of the inhaled anesthetic. MAC is affected by several variables but is unaffected by gender or duration of surgery and anesthesia (see Box 1) (5).
| Box. 1 Factors That Increase or Decrease Anesthetic Requirements |
|---|
|
Factors Increasing MAC |
MAC, Minimum alveolar concentration.
MAC allows potencies to be compared among anesthetics (see Table 1); 1.15% isoflurane is equipotent with 6% desflurane in preventing movement in response to a surgical incision in patients of a similar age and body temperature. Remarkably, MAC values for different inhaled anesthetics are additive. For example, 0.5 MAC of nitrous oxide administered with 0.5 MAC of isoflurane has the same effect as 1 MAC of any inhaled anesthetic in preventing movement in response to incision (reflecting anesthetic-induced inhibition of reflex responses at the level of the spinal cord) (6). The concentration of anesthetic at the brain needed to prevent movement in response to a surgical incision is likely to be larger than the MAC.
| Table. 1 Comparative Characteristics of Inhaled Anesthetics | ||||||
|---|---|---|---|---|---|---|
| Characteristic | Isoflurane | Enflurane | Halothane | Desflurane | Sevoflurane | Nitrous Oxide |
| Partition coefficient | ||||||
| Blood-gas | 1.46 | 1.9 | 2.54 | 0.45 | 0.65 | 0.46 |
| Brain-blood | 1.6 | 1.5 | 1.9 | 1.3 | 1.7 | 1.1 |
| Muscle-blood | 2.9 | 1.7 | 3.4 | 2.0 | 3.1 | 1.2 |
| Fat-blood | 45 | 36 | 51 | 27 | 48 | 2.3 |
| MAC (age 30-55 years) % of 1 atmosphere | 1.15 | 1.63 | 0.76 | 6.0 | 1.85 | 104 |
| Vapor pressure at 20° C (mm Hg) | 240 | 172 | 244 | 669 | 160 | |
| Molecular weight (g) | 184.5 | 184.5 | 197.4 | 168 | 200 | 44 |
| Stable in hydrated CO2 absorbent | Yes | Yes | No(a) | Yes | No(a) | Yes |
| Stable in dehydrated CO2 absorbent |
No | No(ab) | No(b) | No(abc) | Yes | |
| Percent metabolized | 0-0.2 | 15-40 | 0-0.2 | 5-8 | ||
(a) Compound A.
(b) Carbon monoxide.
(c) Severe exothermic reactions reported.
MAC, Minimum alveolar concentration.
The anesthetic dose required to produce amnesia prob-ably has more variability than the MAC. The alveolar concentration of isoflurane preventing recall of a verbal stimulus was 0.20 MAC in 50% and 0.40 MAC in 95% of volunteers (15). Assuming a standard normal distribution of dose-response, the standard deviation in minimum concentration preventing recall is therefore approximately half the mean value (0.1 MAC). Referring to standard normal curves, we can calculate that the concentration needed by 1 in 100,000 subjects with the highest anesthetic requirement would be 4.27 standard deviations (SD) above the mean (i.e., greater than 0.627 MAC) to prevent recall of verbal stimulus. Extrapolation of this value to the context of surgery must be made with caution, however, because the dose required to prevent recall of painful as opposed to verbal stimulation may be considerably larger (16). The ratio of concentration needed to prevent motor response to surgical incision (reflected in MAC) to that required to suppress consciousness and prevent recall differs slightly between individual potent inhaled anesthetics and differs substantially between potent inhaled anesthetics collectively versus nitrous oxide. Volunteers given isoflurane did not exhibit recall given 0.45 MAC of isoflurane, whereas recall did occur with as much as 0.6 MAC of nitrous oxide (17).
Pharmacokinetics of inhaled anesthetics describes their uptake (absorption) from alveoli into the systemic circulation, distribution in the body, and eventual elimination by the lungs or metabolism principally in the liver (Box 2) (18). By controlling the inspired partial pressure (PI) (same as the concentration [%] when referring to the gas phase) of an inhaled anesthetic, a gradient is created such that the anesthetic is delivered from the anesthetic machine to its site of action, the brain. The primary objective of inhaled anesthesia is to achieve a constant and optimal brain partial pressure (Pbr) of the anesthetic.
| Box 2. Factors Determining Partial Pressure Gradients Necessary for Establishment of Anesthesia. |
|---|
Transfer of inhaled anesthetic from anesthetic machine to alveoli
|
The brain and all other tissues equilibrate with the partial pressure of the inhaled anesthetic delivered to them by the arterial blood (Pa). Likewise, the blood equilibrates with the alveolar partial pressure (PA) of the anesthetic:
PA ⇄ Pa ⇄ Pbr
Maintaining a constant and optimal PA becomes an indirect but useful method for controlling the Pbr. The PA of an inhaled anesthetic mirrors its Pbr and is the reason the PA is used as an index of anesthetic depth, a reflection of the rate of induction and recovery from anesthesia, and a measure of equal potency (see earlier discussion under “Relative Potency of Inhaled Anesthetics”). Understanding the factors that determine the PA and the Pbr allows the anesthesia provider to skillfully control and adjust the dose of inhaled anesthetic delivered to the brain.
The PA and ultimately the Pbr of an inhaled anesthetic are determined by input (delivery) into the alveoli minus uptake (loss) of the drug from the alveoli into the pulmonary arterial blood. Input of the inhaled anesthetic depends on the PI, alveolar ventilation ( ˙VA), and characteristics of the anesthetic breathing system. Uptake of the inhaled anesthetic depends on the solubility, cardiac output (CO), and alveolar-to-venous partial pressure difference (PA − Pv). These six factors act simultaneously to determine the PA. Metabolism and percutaneous loss of inhaled anesthetics do not significantly influence PA during induction and maintenance of anesthesia.
Inspired Anesthetic Partial Pressure
A high PI is necessary during initial administration of an inhaled anesthetic. This initial high PI (i.e., input) offsets the impact of uptake into the blood and accelerates induction of anesthesia as reflected by the rate of increase in the PA. This effect of the PI is known as the concentration effect. Clinically, the range of concentrations necessary to produce a concentration effect is probably possible only with nitrous oxide (Fig. 4) (19).

With time, as uptake into the blood decreases, the PI should be decreased to match the decreased anesthetic uptake. Decreasing the PI to match decreasing uptake with time is critical if the anesthesia provider is to achieve the goal of maintaining a constant and optimal Pbr. For example, if the PI were maintained constant with time (input constant), the PA (and depth of anesthesia as reflected by the Pbr) would progressively increase as uptake of the anesthetic into the blood diminished with time.
Second Gas Effect
The second gas effect is a distinct phenomenon that occurs independently of the concentration effect. The ability of the large-volume uptake of one gas (first gas) to accelerate the rate of increase of the PA of a concurrently administered companion gas (second gas) is known as the second gas effect. For example, the initial large volume uptake of nitrous oxide accelerates the uptake of companion gases such as volatile anesthetics and oxygen. The transient increase (about 10%) in PaO2 that accompanies the early phase of nitrous oxide administration reflects the second gas effect of nitrous oxide on oxygen. This increase in PaO2 has been designated alveolar hyperoxygenation. Increased tracheal inflow of all inhaled gases (i.e., first and second gases) and concentration of the second gases in a smaller lung volume (i.e., concentrating effect) because of the high-volume uptake of the first gas are the explanations for the second gas effect. Although the second gas effect is based on proven pharmacokinetic principles, its clinical importance is doubtful.
Alveolar Ventilation
Increased ˙VA, like PI, promotes input of inhaled anesthetics to offset uptake into the blood. The net effect is a more rapid rate of increase in the PA and induction of anesthesia. Predictably, hypoventilation has the opposite effect, acting to slow the induction of anesthesia.
Controlled ventilation of the lungs that results in hyperventilation and decreased venous return accelerates the rate of increase of the PA by virtue of increased input (i.e., increased ˙VA) and decreased uptake (i.e., decreased CO). As a result, the risk of anesthetic overdose may be increased during controlled ventilation of the lungs, and it may be appropriate to decrease the PI of volatile anesthetics when ventilation of the lungs is changed from spontaneous to controlled to maintain the PA similar to that present during spontaneous ventilation.
Another effect of hyperventilation is decreased cerebral blood flow because of the associated decrease in the PaCO2. Conceivably, the impact of increased anesthetic input on the rate of increase of the PA would be offset by decreased delivery of anesthetic to the brain. Theoretically, coronary blood flow may remain unchanged, such that increased anesthetic input produces myocardial depression, and decreased cerebral blood flow prevents a concomitant onset of central nervous system depression.
Anesthetic Breathing System
Characteristics of the anesthetic breathing system that influence the rate of increase of the PA include the volume of the system, solubility of inhaled anesthetics in the rubber or plastic components of the system, and gas inflow from the anesthetic machine. The volume of the anesthetic breathing system acts as a buffer to slow attainment of the PA. High gas inflow from the anesthetic machine negates this buffer effect. Solubility of inhaled anesthetics in the components of the anesthetic breathing system initially slows the rate at which the PA increases. At the conclusion of an anesthetic, reversal of the partial pressure gradient in the anesthetic breathing system results in elution of the anesthetics that slows the rate at which the PA decreases.
Solubility
The solubility of inhaled anesthetics in blood and tissues is denoted by partition coefficients. A partition coefficient is a distribution ratio describing how the inhaled anesthetic distributes itself between two phases at equilibrium (when the partial pressures are identical). For example, a blood-gas partition coefficient of 10 means that the concentration of the inhaled anesthetic is 10 in the blood and 1 in the alveolar gas when the partial pressures of that anesthetic in these two phases are identical. Partition coefficients are temperature dependent. For example, the solubility of a gas in a liquid is increased when the temperature of the liquid decreases. Unless otherwise stated, partition coefficients are given for 37° C.
Blood-Gas Partition Coefficient
High blood solubility means that a large amount of inhaled anesthetic must be dissolved (i.e., undergo uptake) in the blood before equilibrium with the gas phase is reached. The blood can be considered a pharmacologically inactive reservoir, the size of which is determined by the solubility of the anesthetic in the blood. When the blood-gas partition coefficient is high, a large amount of anesthetic must be dissolved in the blood before the Pa equilibrates with the PA (Fig. 5) (18). Clinically, the impact of high blood solubility on the rate of increase of the PA can be offset to some extent by increasing the PI. When blood solubility is low, minimal amounts of the anesthetic have to be dissolved in the blood before equilibrium is reached such that the rate of increase of the PA and that of the Pa and Pbr are rapid (see Fig. 5) (20).

Tissue-Blood Partition Coefficients
Tissue-blood partition coefficients determine the time necessary for equilibration of the tissue with the Pa (see Table 1). This time can be predicted by calculating a time constant (i.e., amount of inhaled anesthetic that can be dissolved in the tissue divided by tissue blood flow) for each tissue. Brain-blood partition coefficients for a volatile anesthetic such as isoflurane result in time constants of about 3 to 4 minutes. Complete equilibration of any tissue, including the brain, with the Pa requires at least three time constants. This is the rationale for maintaining the PA of this volatile anesthetic constant for 10 to 15 minutes before assuming that the Pbr is similar. Time constants for less soluble anesthetics such as nitrous oxide, desflurane, and sevoflurane are about 2 minutes, and complete equilibration is achieved in approximately 6 minutes (i.e., three time constants).
Anesthetic Transfer by Intertissue Diffusion
There is growing evidence that a portion of anesthetic uptake may occur not by blood flow to various tissues, but by direct transfer from tissues with lower to higher affinity for the anesthetic (i.e., from lean to adipose tissues), such as the interface between viscera and omental fat (see “Context-Sensitive Half-Time”). Larger people (21) and animals (22) with presumably greater lean-fat surface area interface show greater uptake of sevoflurane and isoflurane. Transfer to bulk fat by blood flow during an anesthetic of clinically realistic duration (less than 12 to 24 hours) is unlikely to explain these differences, given the relatively small blood flow received by the bulk fat compartment and its relatively large size.
Nitrous Oxide and Methionine Synthase Inactivation
Nitrous oxide is unique among anesthetics by its inactivation of methionine synthase, the enzyme regulating vitamin B12 and folate metabolism. Although impact of the enzyme inactivation may be subtle or subclinical in many patients, those with underlying critical illness or preexisting vitamin B12 deficiency may suffer neurologic or hematologic sequelae. Homocysteine, which requires functional methionine synthase for conversion to methionine, is associated with increased risk of adverse coronary events when present in elevated concentration in the blood (23). Patients receiving nitrous oxide during carotid endarterectomy showed significantly elevated homocysteine levels and frequency of myocardial ischemic episodes compared to patients not receiving nitrous oxide (24).
Nitrous Oxide Transfer to Closed Gas Spaces
The blood-gas partition coefficient of nitrous oxide (0.46) is 34 times greater than that of nitrogen (0.014). This differential solubility means that nitrous oxide can leave the blood to enter an air-filled cavity 34 times more rapidly than nitrogen can leave the cavity to enter the blood. As a result of this preferential transfer of nitrous oxide, the volume or pressure of the air-filled cavity increases. The entrance of nitrous oxide into an air-filled cavity surrounded by a compliant wall (e.g., intestinal gas, pneumothorax, pulmonary blebs, air embolism) causes the gas space to expand. Conversely, entrance of nitrous oxide into an air-filled cavity surrounded by a noncompliant wall (e.g., middle ear, cerebral ventricles, supratentorial subdural space) causes an increase in pressure.
The magnitude of volume or pressure increase in the air-filled cavity is influenced by the PA of nitrous oxide, blood flow to the air-filled cavity, and duration of nitrous oxide administration. In an animal model, inhalation of 75% nitrous oxide doubles the volume of a pneumothorax in 10 minutes (25). The presence of a closed pneumothorax is a contraindication to the administration of nitrous oxide. Decreasing pulmonary compliance during administration of nitrous oxide to a patient with a history of chest trauma (i.e., rib fractures) may reflect nitrous oxide–induced expansion of a previously unrecognized pneumothorax. Likewise, air bubbles associated with venous air embolism expand rapidly when exposed to nitrous oxide. In contrast to the rapid expansion of a pneumothorax or air bubbles (i.e., venous air embolism), the increase in bowel gas volume produced by nitrous oxide is slow. The question of whether to administer nitrous oxide to patients undergoing intra-abdominal surgery is of little importance if the operation is short. Limiting the inhaled concentration of nitrous oxide to 50%, however, may be a prudent recommendation when bowel gas volume is increased (e.g., bowel obstruction) preoperatively. Following this guideline, bowel gas volume at most would double, even during prolonged operations (25).
Cardiac Output
The CO influences uptake into the pulmonary arterial blood and therefore PA by carrying away more or less anesthetic from the alveoli. A high CO (e.g., induced by anxiety) results in more rapid uptake, such that the rate of increase in the PA and the induction of anesthesia are slowed. A low CO (e.g., shock) speeds the rate of increase of the PA because there is less uptake into the blood to oppose input. A common clinical impression is that induction of anesthesia in patients in shock is rapid.
Shunt
A right-to-left intracardiac or intrapulmonary shunt slows the rate of induction of anesthesia. This slowing reflects the dilutional effect of shunted blood containing no anesthetic on the partial pressure of anesthetic in blood coming from ventilated alveoli. A similar mechanism is responsible for the decrease in PaO2 in the presence of a right-to-left shunt.
A left-to-right shunt (e.g., arteriovenous fistula, volatile anesthetic-induced increases in cutaneous blood flow) results in delivery to the lungs of venous blood containing a higher partial pressure of anesthetic than that present in venous blood that has passed through the tissues. As a result, a left-to-right tissue shunt offsets the dilutional effect of a right-to-left shunt on the Pa. The effect of a left-to-right shunt on the rate of increase in the Pa is detectable only if there is the concomitant presence of a right-to-left shunt. Likewise, the dilutional effect of a right-to-left shunt is greatest in the absence of a left-to-right shunt. All factors considered, it seems unlikely that the impact of a right-to-left shunt would be clinically apparent.
Wasted Ventilation
Ventilation of nonperfused alveoli does not influence the rate of induction of anesthesia because a dilutional effect on the Pa is not produced. The principal effect of wasted ventilation is the production of a difference between the PA and Pa of the inhaled anesthetic. A similar mechanism is responsible for the difference often observed between the end-tidal PCO2 and PaCO2.
Alveolar-to-Venous Partial Pressure Differences
The PA – Pv reflects tissue uptake of inhaled anesthetics. Highly perfused tissues (i.e., brain, heart, kidneys, and liver) account for less than 10% of body mass but receive about 75% of the CO (Table 2). As a result, these highly perfused tissues equilibrate rapidly with the Pa. After three time constants (6 to 12 minutes for inhaled anesthetics), about 75% of the returning venous blood is at the same partial pressure as the PA (i.e., narrow PA − Pv). For this reason, uptake of volatile anesthetics from the alveoli is greatly decreased after 6 to 12 minutes, as reflected by a narrowing of the PI − PA difference. After this time, the inhaled concentrations of volatile anesthetics should be decreased to maintain a constant PA in the presence of decreased uptake.
| Table 2. Body Tissue Compartments. | ||
|---|---|---|
| Compartment | Body Mass (% of 70-kg Adult Male) | Blood Flow (% Cardiac Output, 70-kg Adult Male) |
| Vessel-rich group | 10 | 75 |
| Muscle group | 50 | 19 |
| Fat group | 20 | 5 |
| Vessel-poor group | 20 | 1 |
Skeletal muscle and fat represent about 70% of the body mass but receive less than 25% of the CO (Box 3). These tissues continue to act as inactive reservoirs for anesthetic uptake for several hours. Equilibration of fat with inhaled anesthetics in the arterial blood is probably never achieved.
| Box 3. Proposed Mechanisms of Circulatory Effects Produced by Inhaled Anesthetics. |
|---|
| Direct myocardial depression Inhibition of central nervous system and sympathetic nervous system outflow Depression of transmission of impulses through autonomic ganglia Attenuated carotid sinus reflex activity Decreased formation of cyclic adenosine monophosphate Inhibition of calcium reuptake by myocardial sarcoplasmic reticulum Decreased influx of calcium ions through slow channels |
Recovery from anesthesia can be defined as the rate at which the PA decreases with time (Fig. 6) (20). In many respects, recovery is the inverse of induction of anesthesia. For example, ˙VA, solubility, and CO determine the rate at which the PA decreases. After discontinuation of anesthetic administration, elimination of anesthetic occurs by ventilation of the lungs. As the alveolar partial pressure decreases, anesthetic is subsequently transferred from the tissues (including the brain) into the alveoli. Hypoventilation or use of fresh gas flows low enough to permit rebreathing of anesthetic will lead to transfer of anesthetic back into the tissues (including the brain), delaying patient recovery.

How Does Recovery Differ From Induction of Anesthesia?
Recovery from anesthesia differs from induction of anesthesia with respect to the absence of a concentration effect on recovery (the PI cannot be less than zero), the variable tissue concentrations of anesthetics at the start of recovery, and the potential importance of metabolism on the rate of decrease in the PA.
Tissue Concentrations
Tissue concentrations of inhaled anesthetics serve as a reservoir to maintain the PA when the partial pressure gradient is reversed by decreasing the PI to or near zero at the conclusion of anesthesia. The impact of tissue storage depends on the duration of anesthesia and solubility of the anesthetics in various tissue compartments. For example, time to recovery is prolonged in proportion to the duration of anesthesia for a soluble anesthetic (e.g., isoflurane), whereas the impact of duration of administration on time to recovery is minimal with poorly soluble anesthetics (e.g., sevoflurane, desflurane) (Fig 7) (1). The variable concentrations of anesthetics in different tissues at the conclusion of anesthesia contrasts with induction of anesthesia, when all tissues initially have the same zero concentration of anesthetic.

Metabolism
An important difference between induction of anesthesia and recovery from anesthesia is the potential impact of metabolism on the rate of decrease in the PA at the conclusion of anesthesia. In this regard, metabolism is a principal determinant of the rate of decrease in the PA of the highly lipid-soluble methoxyflurane. Metabolism and ˙VA are equally important in the rate of decrease in the PA of halothane, whereas the rate of decrease in the PA of less lipid-soluble isoflurane, desflurane, and sevoflurane principally results from ˙VA (26).
Context-Sensitive Half-Time
The pharmacokinetics of the elimination of inhaled anesthetics depends on the length of administration (the “context”) and the solubility of the inhaled anesthetic in blood and tissues. As with intravenous anesthetics, it is possible to use computer simulations to determine context-sensitive decrement times for volatile anesthetics (the time required to decrease in anesthetic concentrations in the central nervous system to a fraction of that given from a starting point of interest). The kinetic modeling is based upon presence of each tissue compartment within the body (i.e., blood, vessel-rich group, muscle, fat), the relative size of each compartment, the proportional blood flow received by each compartment, and the solubility of each specific anesthetic in the tissue composing the compartment. During anesthetic administration, equilibration implies continued uptake of anesthetic until tissue concentration becomes almost as great as alveolar concentration. Equilibration of anesthetic concentration between the alveoli and a small (less than 10% body mass) compartment with high blood flow (i.e., heart, kidneys, brain) occurs within a relatively short period of time (10 to 15 minutes). Conversely, anesthetic equilibration in larger compartments with lesser proportional blood flow (i.e., skeletal muscle and bulk fat) occurs over a longer period of time (hours), as anesthetic uptake continues. The time needed for a 50% decrease in anesthetic concentrations of isoflurane, desflurane, and sevoflurane is less than 5 minutes and does not increase significantly with increasing duration of anesthesia (27). Presumably, this is a reflection of the initial phase of elimination, which is primarily a function of ˙VA. Determination of other decrement times (≥80%) reveals larger differences between various inhaled anesthetics, especially as anesthetic duration becomes longer (Fig. 8). Simulation may underestimate the uptake of anesthesia, especially with more soluble anesthetics, because it does not account for anesthetic transferred from lean to fatty tissues by intertissue diffusion.

Elimination of all but small amounts of anesthetics (smaller than needed for patients to follow commands) must take place before a patient regains coordinated protective functions, such as the ability to swallow and breathe effectively. Surgical patients given longer anesthesia and a more soluble anesthetic (sevoflurane compared to desflurane) required a longer time interval between awakening and regaining the ability to swallow effectively (22). Awake subjects given small concentrations of sevoflurane and isoflurane demonstrate pharyngeal discoordination (28) and diminished chemical ventilatory drive (29).
Diffusion Hypoxia
Diffusion hypoxia may occur at the conclusion of nitrous oxide administration if patients are allowed to inhale room air. The initial high-volume outpouring of nitrous oxide from the blood into the alveoli when inhalation of this gas is discontinued can so dilute the PAO2 that the PaO2 decreases. The occurrence of diffusion hypoxia is prevented by filling the patient’s lungs with oxygen at the conclusion of nitrous oxide administration.
AnaConDa (Anaesthetic Conserving Device, Sedana Medical AB, Uppsala, Sweden) is a tool that facilitates delivery of inhaled anesthetic (isoflurane, sevoflurane) in the intensive care unit (ICU). Liquid anesthetic is delivered via syringe pump to a chamber that attaches to the breathing circuit, between the endotracheal tube and the Y-piece. The syringe delivers liquid anesthetic at a very slow rate to a porous plastic rod inside the chamber, where the liquid evaporates and mixes with fresh gas flowing from the inspiratory limb of the circuit. Exhaled gas is routed through a charcoal filter that absorbs and reclaims approximately 90% of the exhaled anesthetic. Subsequent inspiratory flow is routed through the charcoal filter, where absorbed anesthetic remixes with the fresh gas.
There is growing interest for the use of potent inhaled anesthetics outside the operating room for postsurgical patients in the ICU, and an increasing accumulation of evidence that doing so is feasible and possibly advantageous (30,31). However, challenges to the use of inhaled anesthetic in the ICU setting include increased dead space and work of breathing due to the interposition of the anesthetic delivery device between the tracheal tube and circuit; loss of anesthetic to the environment during frequent tracheal tube suctioning; and questionable availability of appropriate equipment and caregivers with sufficient knowledge and technical expertise in the delivery of inhaled anesthetic.
Equipotent concentrations of inhaled anesthetics have similar circulatory effects, especially during the maintenance of anesthesia in human volunteers (see Box 3) (32). However, patients undergoing surgery may respond differently from healthy volunteers. For example, factors such as coexisting disease, extremes of age, nonoptimal intravascular volume status, presence of surgical stimulation, and concurrent drugs may alter, attenuate, or exaggerate the responses expected based on data obtained from healthy volunteers.
| Box. 3 Proposed Mechanisms of Circulatory Effects Produced by Inhaled Anesthetics |
|---|
| Direct myocardial depression Inhibition of central nervous system and sympathetic nervous system outflow Depression of transmission of impulses through autonomic ganglia Attenuated carotid sinus reflex activity Decreased formation of cyclic adenosine monophosphate Inhibition of calcium reuptake by myocardial sarcoplasmic reticulum Decreased influx of calcium ions through slow channels |
Responses During Maintenance of Anesthesia
Mean Arterial Pressure
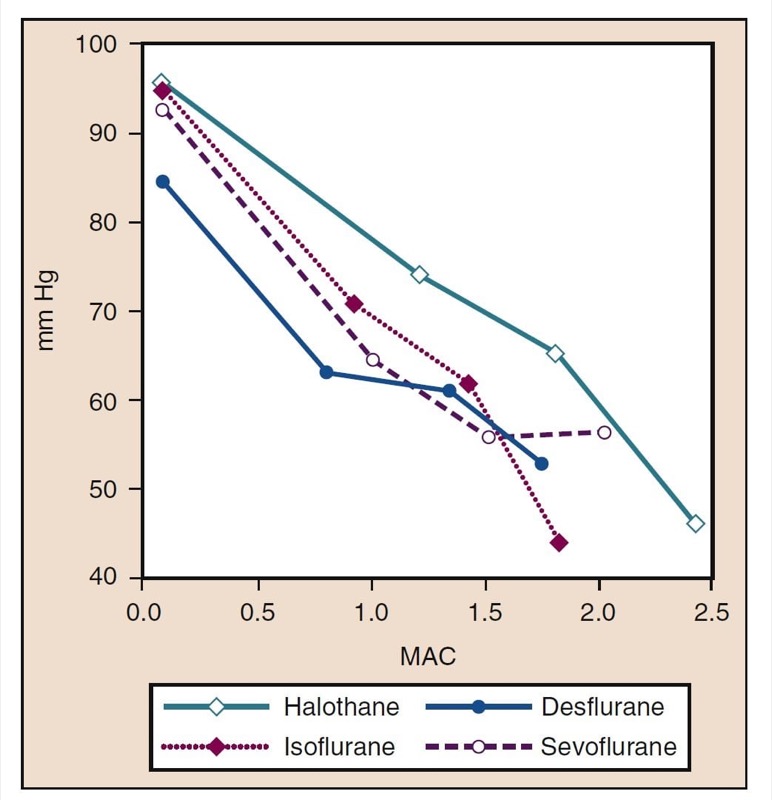
Mean arterial pressure (MAP) decreases with increasing concentrations of desflurane, sevoflurane, isoflurane, halothane, and enflurane in a dose-dependent manner (see Fig. 8) (17,18). With the exception of halothane, the decrease in MAP primarily reflects a decrease in systemic vascular resistance (SVR) versus a decrease in CO (Figs. 9 and 10) (32,33). In contrast, halothane decreases MAP partly or entirely by decreasing CO, whereas SVR is relatively unchanged. These findings are supported by measurements of SVR in patients receiving desflurane, sevoflurane, and isoflurane while undergoing cardiopulmonary bypass perfusion. The dose-related decrease in SVR is minimized by substitution of nitrous oxide for a portion of the volatile drug (Fig. 11) (34). Nitrous oxide, in contrast to the other inhaled anesthetics, causes unchanged or mildly increased MAP (Box 4).



| Box 4. Evidence of a Sympathomimetic Effect of Nitrous Oxide Administered Alone or Added to Unchanging Concentrations of Volatile Anesthetics. |
|---|
| Diaphoresis Increased body temperature Increased plasma concentrations of catecholamines Increased right atrial pressure Mydriasis Vasoconstriction in the systemic and pulmonary circulations |
Heart Rate
Stepwise increases in the delivered concentrations of isoflurane, desflurane, and sevoflurane increase heart rates in patients and volunteers, although at different concentrations (Fig. 12) (33). At concentrations as low as 0.25 MAC, isoflurane causes a linear, dose-dependent heart rate increase. Heart rate increases minimally with desflurane concentrations of less than 1 MAC. When desflurane concentrations are increased above 1 MAC, the heart rate accelerates in a linear, dose-dependent manner. In contrast to desflurane and isoflurane, heart rate in the presence of sevoflurane does not increase until the concentration exceeds 1.5 MAC (35). However, induction with 8% sevoflurane (i.e., single-breath induction) causes tachycardia in both children and adult patients undergoing controlled hyperventilation. This tachycardia may result from sympathetic nervous system stimulation associated with epileptiform brain activity (36).

The tendency for desflurane to stimulate the circulation (i.e., increase MAP and heart rate) is attenuated with administration of β-adrenergic blocker (esmolol), opioid (fentanyl), and passage of time (10 to 15 minutes) during maintenance of anesthesia (also see “Circulatory Effects With Rapid Concentration Increase”). The dose-related increase in heart rate seen with desflurane concentrations more than 1 MAC is not attenuated by substitution of some of the desflurane by nitrous oxide. Isoflurane, sevoflurane, and desflurane, like halothane, diminish baroreceptor responses in a concentration-dependent manner. The transient increase in heart rate above 1 MAC seen with desflurane results from sympathetic nervous system stimulation rather than a reflex baroreceptor activity response to decreased MAP (37).
Cardiac Index
The cardiac index is minimally influenced by administration of desflurane, sevoflurane, or isoflurane over a wide range of concentrations in healthy young adults (see Fig. 10) (32). Transesophageal echocardiography data show that desflurane produces minor increases in the ejection fraction and left ventricular velocity of circumferential shortening compared with awake measurements.
Circulatory Effects With Rapid Concentration Increase
At concentrations of less than 1 MAC, desflurane does not increase heart rate or MAP. However, abrupt increases in inspired desflurane concentrations above 1 MAC cause transient circulatory stimulation in the absence of opioids, adrenergic blockers, or other analgesic adjuncts (Fig. 13) (38). To a lesser extent, isoflurane has a similar capability to evoke increases in heart rate and blood pressure. Accompanying the hemodynamic stimulation seen with abrupt increased concentrations of desflurane and isoflurane are increases in plasma epinephrine and norepinephrine concentrations and sympathetic nervous system activity. An abrupt increase in the inspired sevoflurane concentration from 1 MAC to 1.5 MAC is associated with a slight decrease in heart rate.

A stepwise increase in end-tidal desflurane concentration from 4% to 8% within 1 minute may result in a doubling of the heart rate and blood pressure above baseline. Administration of small doses of opioids, clonidine, or esmolol profoundly attenuates the heart rate and blood pressure responses to the stepwise increase in desflurane concentration. Repetition of the rapid increase in end-tidal desflurane concentration from 4% to 8% after 30 minutes results in minimal changes of the heart rate and MAP, suggesting that the receptors mediating these circulatory changes adapt to repeated stimulation. Circulatory stimulation is not seen with abrupt increases in the concentrations of sevoflurane, halothane, or enflurane up to 2 MAC (see Fig. 13) (38).
Sevoflurane and halothane are frequently given via inhalation to induce anesthesia because of their lack of pungency. Induction of anesthesia in children with halothane, but not sevoflurane, depresses myocardial contractility. In adults, maintenance of anesthesia with 1 MAC of sevoflurane or halothane with 67% nitrous oxide decreases myocardial contractility. In adults, sevoflurane can transiently increase heart rate when controlled ventilation is used.
Administration With Nitrous Oxide and Oxygen Versus 100% Oxygen
Desflurane, isoflurane, and sevoflurane, administered with nitrous oxide and oxygen, decrease the MAP, SVR, cardiac index, and left ventricular stroke work index (LVSWI) in a dose-dependent manner, whereas heart rate, pulmonary artery pressure, and central venous pressure increase, consistent with the findings in which each volatile anesthetic is administered in oxygen alone (see Fig. 11) (32,33). Direct comparison reveals a more pronounced diminution of MAP, SVR, cardiac index, and LVSWI and a more rapid heart rate and larger CO when desflurane is administered in oxygen rather than in nitrous oxide at roughly equivalent MAC multiples (34).
Myocardial Conduction and Dysrhythmogenicity
Isoflurane, sevoflurane, and desflurane do not predispose the heart to premature ventricular extrasystoles (39). In contrast, halothane does sensitize the myocardium to premature ventricular extrasystoles, especially in the presence of catecholamines; this relationship is exaggerated with hypercarbia. Inhaled anesthetics probably suppress ventricular dysrhythmias during myocardial ischemia by prolonging the effective refractory period.
The choice of inhaled anesthetic influences the occurrence of reflex bradydysrhythmias that may result from vagal stimulation. Children anesthetized with sevoflurane, compared with halothane, exhibit fewer episodes of decreased heart rate or sinus node arrest in response to surgical traction on the ocular muscles.
QT Interval
Inhaled anesthetics prolong the QT interval on the electrocardiogram (40). Although each anesthetic’s relative tendency to prolong the QT interval has not been compared systematically, sevoflurane should be avoided in patients with known congenital long QT syndrome (LQTS). Although sevoflurane and propofol anesthetics cause QT interval prolongation in children, neither anesthetic increases transmural dispersion of repolarization, a measure of the heterogeneous rates of repolarization of myocardial cells during phases 2 and 3 of the action potential (41). The clinical significance of QT prolongation with sevoflurane and other inhaled anesthetics in susceptible patients is unclear. In patients with LQTS, β-adrenergic blockade is the mainstay of therapy. Patients with known LQTS have been safely anesthetized with all modern inhaled anesthetics when concurrently receiving β-adrenergic blocking drugs. Numerous malignant intraoperative arrhythmias have occurred in patients undergoing anesthesia with halothane that were subsequently attributed to undiagnosed LQTS, and none of the patients had received β-blocking drugs (40).
Patients With Coronary Artery Disease
Numerous studies in patients undergoing coronary artery bypass surgery or at risk for coronary artery disease have failed to demonstrate a difference in outcome between groups receiving inhaled (i.e., desflurane) versus intravenously administered (i.e., fentanyl or sufentanil) anesthetic techniques or between groups receiving one inhaled anesthetic versus another (i.e., desflurane vs. isoflurane or sevoflurane vs. isoflurane) (42). Concerns that isoflurane’s capacity to dilate small-diameter coronary arteries might cause coronary steal, in which a patient with susceptible anatomy might develop regional myocardial ischemia as a result of coronary vasodilatation, were not valid. Volatile anesthetics instead exert a protective effect on the heart, limiting the area of myocardial injury and preserving function after exposure to ischemic insult.
Anesthetic Preconditioning
The explanation for the protective benefits of volatile anesthetics against myocardial ischemia is called anesthetic preconditioning, and it is not explained by favorable alteration of myocardial oxygen supply-demand ratio. Evidence suggests that volatile anesthetics exert protective effects on the myocardium in the setting of compromised regional perfusion. In patients undergoing coronary artery bypass graft (CABG) surgery, maintenance with 0.2 to 1 MAC of desflurane or sevoflurane decreased the incidence of abnormally increased troponin levels compared with patients receiving propofol (43). Sevoflurane administered for the entire duration of CABG surgery versus prebypass or postbypass administration resulted in a less frequent rate of postoperative myocardial infarction compared with sevoflurane administered only in the prebypass or postbypass period, and prebypass or postbypass administration resulted in a smaller risk of myocardial infarction compared with propofol anesthesia (44).
Mechanisms of Ischemic Preconditioning
Ischemic preconditioning is a fundamental protective mechanism present in all tissues in all species. In is chemic preconditioning, exposure to single or multiple brief episodes of ischemia can confer a protective effect on the myocardium against reversible or irreversible injury with a subsequent prolonged ischemic insult. There are two distinct periods after a brief ischemic episode during which the myocardium is protected. The first period occurs for 1 to 2 hours after the conditioning episode and then dissipates. In the second period, the benefit reappears 24 hours later and can last as long as 3 days. The opening of mitochondrial adenosine triphosphate (ATP)–sensitive potassium channels (KATP) is the crucial event that confers the protective activity, resulting from binding of various ligands to G protein–coupled receptors. Volatile anesthetics enhance ischemic preconditioning or provide direct myocardial protection, and the KATP channels play a central role in their protective effects (45).
Inhaled anesthetics increase the frequency of breathing and decrease tidal volume as anesthetic concentration increases. Although minute ventilation is relatively preserved, the decreased tidal volume leads to a relatively greater proportion of dead space ventilation relative to alveolar ventilation. Gas exchange becomes progressively less efficient at deeper levels of anesthesia, and PaCO2 increases proportionally with anesthetic concentration (Fig. 14) (1). Effects are similar among potent anesthetics at given MAC multiples. Substitution of nitrous oxide (60%) for an equivalent portion of volatile anesthetic may attenuate the increase in PaCO2 at deeper levels of anesthesia.

Volunteers and patients breathing desflurane (and other volatile anesthetics) show a dose-related blunting of carbon dioxide responsiveness, which leads to apnea in subjects receiving 1.7 MAC of desflurane in oxygen (Fig 15) (1). Compared with volunteers, the blunting of ventilation with inhaled anesthetics may be less pronounced in patients undergoing surgery, reflecting the stimulatory effect of surgery on breathing (Fig. 16) (1). Volatile anesthetics all blunt the ventilatory stimulation evoked by arterial hypoxemia (46).


Chest Wall Changes
Inhaled anesthetics contribute to conformational changes in the chest wall that may influence ventilatory mechanics. Cephalad displacement of the diaphragm and inward displacement of the rib cage occur from enhanced expiratory muscle activity, and the net result contributes to reduction in functional residual capacity. Atelectasis occurs preferentially in the dependent areas of the lung and occurs to a greater extent when spontaneous ventilation is permitted.
Hypoxic Pulmonary Vasoconstriction
Inhaled anesthetics alter pulmonary blood flow, but inhibition of hypoxic pulmonary vasoconstriction is minimal. For example, arterial oxygenation is similar in patients undergoing one-lung ventilation with isoflurane versus desflurane anesthesia and sevoflurane versus propofol anesthesia (47).
Airway Resistance
In the absence of bronchoconstriction, bronchodilating effects of inhaled anesthetics are small. In volunteers, isoflurane, halothane, and sevoflurane, but not nitrous oxide and thiopental, decrease respiratory systemic resistance after tracheal intubation. In nonsmokers, airway resistance shows no change after tracheal intubation and desflurane anesthesia compared with a modest decrease with sevoflurane, whereas smokers show a mild, transient increase in airway resistance after tracheal intubation and desflurane anesthesia (48). Some or all of the changes in airway resistance may be mediated by changes in gas density.
Airway Irritant Effects
Inhaled anesthetics differ in their capacity to irritate airways (i.e., pungency). Sevoflurane, halothane, and nitrous oxide are nonpungent and cause minimal or no irritation over a broad range of concentrations. Desflurane and isoflurane are pungent, and they can irritate the airways in concentrations exceeding 1 MAC, particularly in the absence of intravenous medications (e.g., opioids, sedative-hypnotics) that decrease the perception of pungency.
Sevoflurane or halothane is selected most frequently when an inhaled induction of anesthesia is desired. However, desflurane and isoflurane may be administered to surgical patients by means of a laryngeal mask airway without a greater incidence of airway irritation (e.g., coughing, breath-holding, laryngospasm, arterial oxygen desaturation) compared with sevoflurane or propofol, because maintenance usually does not require a concentration in excess of 1 MAC (i.e., nonirritating concentrations) (49).
Cerebral Blood Flow
Nitrous oxide administered without volatile anesthetics causes cerebral vasodilatation and increases cerebral blood flow. The cerebral metabolic rate for oxygen (CMRO2) increases modestly. Coadministration of opioids, barbiturates, or propofol (but not ketamine) counteracts these effects (50). Inhaled anesthetics do not abolish cerebral vascular responsiveness to changes in PaCO2 (51).
Halothane, isoflurane, sevoflurane, and desflurane decrease CMRO2. In normocapnic humans, these volatile anesthetics cause cerebral vasodilatation at concentrations above 0.6 MAC. There is a biphasic dose-dependent effect on cerebral blood flow. At 0.5 MAC, the decrease in CMRO2 counteracts the vasodilatation such that cerebral blood flow does not change significantly. At concentrations in excess of 1 MAC, vasodilating effects predominate and cerebral blood flow increases, especially if systemic blood pressure is maintained at awake levels. The cerebral blood flow increase is relatively greater with halothane compared with isoflurane, sevoflurane, or desflurane.
Intracranial Pressure
Intracranial pressure increases with all of the volatile anesthetics at doses more than 1 MAC, and autoregulation (i.e., adaptive mechanism normalizing cerebral blood flow over a wide range of systemic arterial pressures in awake patients) is impaired at concentrations of less than 1 MAC. Patients undergoing craniotomy for supratentorial tumors who receive 1 MAC of isoflurane or desflurane show decreased cerebral perfusion pressure and an arteriovenous oxygen difference for oxygen but no change in intracranial pressure (52). However, patients undergoing pituitary tumor resection who receive 1 MAC of desflurane, isoflurane, or sevoflurane show small increases in intracranial pressure and decreased cerebral blood flow. Neurosurgical patients receiving 50% nitrous oxide plus 0.5 MAC of desflurane or isoflurane apparently have more brain relaxation than those receiving 1 MAC of desflurane or isoflurane without nitrous oxide. Inhaled anesthetics do not abolish cerebral vascular responsiveness to changes in PaCO2 (51).
Evoked Potentials
All volatile anesthetics and nitrous oxide depress the amplitude and increase the latency of somatosensory evoked potentials in a dose-dependent manner. Evoked potentials may be abolished at 1 MAC of volatile anesthetic alone or above 0.5 MAC administered with 50% nitrous oxide. Low concentrations of volatile anesthetics (0.2 to 0.3 MAC) can decrease the reliability of motor evoked potential monitoring, although the impact can be partially overcome by the use of multipulse stimuli (53).
Electroencephalographic Effects
Volatile anesthetics cause characteristic, dose-dependent changes in the EEG. Increasing depth of anesthesia from the awake state is characterized by increased amplitude and synchrony. Periods of electrical silence begin to occupy a greater proportion of the time as depth increases (i.e., burst suppression). This isoelectric pattern predominates on the EEG within the range of 1.5 to 2.0 MAC.
Sevoflurane and enflurane may be associated with epileptiform activity on the EEG, especially at higher concentrations or when controlled hyperventilation is instituted. Seizure-like activity has been reported in children during sevoflurane induction, but the clinical implications of these observations are not clear (54).
Volatile anesthetics produce dose-related skeletal muscle relaxation and enhance the activity of neuromuscular blocking drugs. Enhancement of the relaxant effect of rocuronium is more intense with anesthesia from desflurane than with sevoflurane or isoflurane, although all volatile anesthetics enhance skeletal muscle relaxation compared with intravenous anesthetics (e.g., propofol plus fentanyl). Elimination of volatile anesthetic enhances recovery from neuromuscular blockade. A decrease in the desflurane concentration to 0.25 MAC facilitates reversal of neuromuscular block after vecuronium administration more than an equipotent decrease in isoflurane concentration.
Malignant hyperthermia (MH) continues to be a life-threatening complication of anesthesia. It is an inherited disorder of increased skeletal muscle metabolism that is triggered with the administration of a volatile anesthetic, especially halothane and/or succinylcholine. Although all potent inhaled volatile anesthetics also have the potential to trigger MH, studies with desflurane, sevoflurane, and possibly isoflurane suggest less risk than that of halothane. Males appear to be more susceptible to developing a clinical MH episode than females (55,56). The pediatric population accounts for 52.1% of all MH reactions (57,58). Signs of MH are related to increased metabolism and include tachycardia, increased end-tidal carbon dioxide levels, muscle rigidity, and increased temperature.
More recent cases of MH are less severe due to improved diagnostic awareness, early detection through end-expired carbon dioxide, less use of potent anesthetic triggers, and the administration of drugs that attenuate fulminant episodes of MH.
The key aspects of management include discontinuation of volatile anesthetics and succinylcholine, immediate administration of intravenous dantrolene, and treatment of potentially life-threatening electrolyte abnormalities such as hyperkalemia. The Malignant Hyperthermia Association of the United States (MHAUS) provides detailed treatment recommendations on its website
Hepatic injury after anesthesia may be categorized as severe (immune-mediated) or mild (59).
Immune-Mediated Liver Injury
Severe hepatic injury may follow anesthesia with halothane, isoflurane, sevoflurane, or desflurane. This severe form involves massive hepatic necrosis that can lead to death or necessitate liver transplantation. The mechanism for this severe injury is immunologic, requiring prior exposure to a volatile anesthetic. Halothane, isoflurane, and desflurane all undergo oxidative metabolism by cytochrome P-450 enzymes to produce trifluoroacetate. The trifluoroacetate can bind covalently to hepatocyte proteins. The trifluoroacetyl-hepatocyte moieties can act as haptens, which the body recognizes as foreign and to which the immune system forms antibodies. Subsequent exposure to any anesthetic capable of producing trifluoroacetate may provoke an immune response, leading to severe hepatic necrosis (60). Sevoflurane is metabolized to hexafluoroisopropanol, a compound that does not have the equivalent antigenic behavior as trifluoroacetate (61).
Mild Liver Injury
A clinically mild form of liver injury may follow administration of halothane. The main characteristic of this more common entity is modest elevation of serum transaminase levels. This mild form of liver injury is thought to be mediated by reductive metabolism of halothane and may be more likely to occur after concomitant decreases in hepatic blood flow and associated reductions in oxygen delivery to the liver.
History of Prior Anesthesia-Related Hepatic Dysfunction
Although volatile anesthetics are frequently not given in patients who have experienced unexplained symptoms of hepatic dysfunction after inhaled anesthesia on a previous occasion, volatile anesthetics are probably not harmful to patients with preexisting hepatic disease unrelated to anesthesia.
Methoxyflurane was the first nonflammable volatile anesthetic introduced to clinical practice. Its use was associated with renal injury. Subsequent investigation suggests that its extensive metabolism, specifically to inorganic fluoride and dichloroacetic acid from O-demethylation, is probably responsible for the injury. Yet, fluoride production from metabolism of other potent inhaled anesthetics, specifically sevoflurane, shows no association with renal injury (62).
1. Eger EI. Desflurane (Suprane): A Com-pendium and Reference. Nutley, NJ: Anaquest; 1993.
2. Eger EI II. History of Modern Inhaled An-esthetics: The Pharmacology of Inhaled Anesthetics. San Antonio, TX: Danne-miller Memorial Educational Foundation; 2000.
3. Eger II EI. New inhaled anesthetics. Anesthesiology. 1994;80:906–922.
4. Eger EI II, Koblin DD, Harris RA, et al. Hypothesis: inhaled anesthetics produce immobility and amnesia by different mechanisms at different sites. Anesth Analg. 1997;84:915–918.
5. Quasha AL, Eger EI II, Tinker JH, et al. Determination and application of MAC. Anesthesiology. 1980;53(4):315–334.
6. Rampil IJ. Anesthetic potency is not altered after hypothermic spinal cord transection in rats. Anesthesiology. 1994;80:606–610.
7. Guo TZ, Poree L, Golden W, et al. Antinociceptive response to nitrous oxide is mediated by supraspinal opiate and spinal alpha 2 adrenergic receptors in the rat. Anesthesiology. 1996;85(4):846–852.
8. Sawamura S, Kingery WS, Davies MF, et al. Antinociceptive action of nitrous oxide is mediated by stimulation of noradrenergic neurons in the brainstem and activation of [alpha]2B adrenoceptors. J Neurosci. 2000;20(24):9242–9251.
9. Weiskopf RB, Sampson D, Moore MA. The desflurane (Tec 6) vaporizer: de-sign, design considerations and performance evaluation. Br J Anaesth. 1994;72(4):474–479.
10. Brockwell RC, Andrews JJ. Vaporizers (in delivery systems for inhaled anesthetics). In: Barash PG, Cullen BF, Stoelting RK, Cahallan M, eds. Clinical Anesthesia. 6th ed. Philadelphia: Lippincott Williams & Wilkins; 2009:667–669.
11. Carpenter RL, Eger EI II, Johnson BH, et al. The extent of metabolism of inhaled anesthetics in humans. Anesthesiology. 1986;65:201–205.
12. Wissing H, Kuhn I, Warnken U, et al. Carbon monoxide production from desflurane, enflurane, halothane, isoflurane, and sevoflurane with dry soda lime. Anesthesiology. 2001;95:1205–1212.
13. Laster MJ, Roth P, Eger EI II. Fires from the interaction of anesthetics with desiccated absorbent. Anesth Analg. 2004;99:769–774.
14. Wu J, Previte JP, Adler E, et al. Spontaneous ignition, explosion, and fire with sevoflurane and barium hydroxide lime. Anesthesiology. 2004;101: 534–537.
15. Chortkoff BS, Bennett HL, Eger EI II. Subanesthetic concentrations of isoflurane suppress learning as defined by the category-example task. Anesthesiology. 1993;79(1):16–22.
16. Sonner JM, Gong D, Eger EI II. Natu-rally occurring variability in anesthetic potency among inbred mouse strains. Anesth Analg. 2000;91(3):720–726.
17. Dwyer R, Bennett HL, Eger EI II, et al. Effects of isoflurane and nitrous oxide in subanesthetic concentrations on memory and responsiveness in volunteers. Anesthesiology. 1992;77(5):888–898.
18. Eger EI II. Uptake of inhaled anesthetics: the alveolar to inspired anesthetic difference. In: Eger EI II, ed. Anesthetic Uptake and Action. Baltimore: Williams & Wilkins; 1974:77–96.
19. Eger EI. Effect of inspired anesthetic concentration on the rate of rise of alveolar concentration. Anesthesiology. 1963;24:153–157.
20. Yasuda N, Lockhart SH, Eger EI II, et al. Comparison of kinetics of sevoflurane and isoflurane in humans. Anesth Analg. 1992;72:316–324.
21. McKay RE, Malhotra A, Cakmakkaya OS, et al. Effect of increased body mass index and anaesthetic duration on recovery of protective airway reflexes after sevoflurane vs desflurane. Br J Anaesth. 2010;104:175–182.
22. Wahrenbrock EA, Eger EI II, Laravuso RB, et al. Anesthetic uptake—of mice and men (and whales). Anesthesiology. 1974;40(1):19–23.
23. Aronow WS, Ahn C. Increased plasma homocysteine is an independent predictor of new coronary events in older persons. Am J Cardiol. 2000;86(3):346–347.
24. Badner NH, Beattie WS, Freeman D, et al. Nitrous oxide-induced increased homocysteine concentrations are associated with increased postoperative myocardial ischemia in patients undergoing carotid endarterectomy. Anesth Analg. 2000;91(5):1073–1079.
25. Eger EI II, Saidman JL. Hazards of nitrous oxide anesthesia in bowel obstruction and pneumothorax. Anesthesiology. 1965;26:61–66.
26. Ryan S, Neilsen CJ. Global warming potential of inhaled anesthetics: application to clinical use. Anesth Analg. 2010;111:92–98.
27. Bailey JM. Context-sensitive half-times and other decrement times of inhaled anesthetics. Anesth Analg. 1997;85: 681–686.
28. Sundman E, Witt H, Sandin R, et al. Pharyngeal function and airway protection during subhypnotic concentrations of propofol, isoflurane, and sevoflurane: volunteers examined by pharyngeal videoradiography and simultaneous manometry. Anesthesiology. 2001;95(5):1125–1132.
29. Dahan A, Teppema LJ. Influence of anaesthesia and analgesia on the control of breathing. Br J Anaesth. 2003;91:40–49.
30. Bellgardt M, Bomberg H, Herzog-Niescery J, et al. Survival after long-term isoflurane sedation as opposed to intravenous sedation in critically ill surgical patients: retrospective analysis. Eur J Anaesthesiol. 2016;33(1):6–13.
31. Sackey PV, Martling CR, Granath F, Radell PJ. Prolonged isoflurane sedation of intensive care unit patients with the anesthetic conserving device. Crit Care Med. 2004;32(11):2241–2246.
32. Cahalan MK. Hemodynamic Effects of Inhaled Anesthetics. Review Courses. Cleveland: International Anesthesia Research Society; 1996.
33. Cahalan MK, Weiskopf RB, Eger EI II, et al. Hemodynamic effects of desflurane/nitrous oxide anesthesia in volunteers. Anesth Analg. 1991;73:157–164.
34. Eger EI. Isoflurane (Forane): A Compendium and Reference. Madison, WI: Ohio Medical Products; 1985.
35. Malan TP Jr, DiNardo JA, Isner RJ, et al. Cardiovascular effects of sevoflurane compared with those of isoflurane in volunteers. Anesthesiology. 1995;83:918–928.
36. Yli-Hankala A, Vakkuri AP, Sarkela M, et al. Epileptiform electroencephalogram during mask induction of anesthesia with sevoflurane. Anesthesiology. 1999;91:1596.
37. Ebert TJ, Perez F, Uhrich TD, et al. Desflurane-mediated sympathetic activation occurs in humans despite preventing hypotension and baroreceptor unloading. Anesthesiology. 1998;88:1227–1232.
38. Ebert TJ, Muzi M, Lopatka CW. Neuro-circulatory responses to sevoflurane in humans: a comparison to desflurane. Anesthesiology. 1995;83:88–95.
39. Navarro R, Weiskopf RB, Moore MA, et al. Humans anesthetized with sevoflurane or isoflurane have similar arrhythmic response to epinephrine. Anesthesiology. 1994;80:545–549.
40. Booker PD, Whyte SD, Ladusans EJ. Long QT syndrome and anaesthesia. Br J Anaesth. 2003;90:349–366.
41. Whyte SD, Booker PD, Buckley DG. The effects of propofol and sevoflurane on the QT interval and transmural dispersion of repolarization in children. Anesth Analg. 2005;100:71–77.
42. Grundmann U, Muler M, Kleinschmidt S, et al. Cardiovascular effects of desflurane and isoflurane in patients with coronary artery disease. Acta Anaesthesiol Scand. 1996;40:1101–1107.
43. DeHert SG, Cromheecke S, ten Broecke PW, et al. Effects of propofol, desflurane, and sevoflurane on recovery of myocardial function after coronary surgery in elderly high-risk patients. Anesthesiology. 2003;99:314–323.
44. DeHert SG, Van der Linden PJ, Crom-heecke S, et al. Cardioprotective properties of sevoflurane in patients undergoing coronary surgery and cardiopulmonary bypass are related to the modalities of its administration. Anesthesiology. 2004;101:299–310.
45. Zaugg M, Lucchinetti E, Spahn D, et al. Volatile anesthetics mimic cardiac preconditioning by priming the activation of the mitoKATP channels via multiple signaling pathways. Anesthesiology. 2002;97:4–14.
46. Sjögren D, Lindahl SG, Sollevi A. Ventilatory responses to acute and sustained hypoxia during isoflurane anesthesia. Anesth Analg. 1998;86:403–409.
47. Beck DH, Doepfmer UR, Sinemus C, et al. Effects of sevoflurane and propofol on pulmonary shunt fraction during one-lung ventilation for thoracic surgery. Br J Anaesth. 2001;86:38–43.
48. Goff MJ, Arain SR, Ficke DJ, et al. Absence of bronchodilation during desflurane anesthesia: a comparison to sevoflurane and thiopental. Anesthesiology. 2000;93:404–408.
49. Eshima R, Maurer A, King T, et al. A comparison of upper airway responses during desflurane and sevoflurane administration via a laryngeal mask airway. Anesth Analg. 2003;96:701–705.
50. Petersen KD, Landsfeldt U, Cold GE, et al. Intracranial pressure and cerebral hemodynamics in patients with cerebral tumors: a randomized prospective study of patients subjected to craniotomy in propofol-fentanyl, isoflurane-fentanyl, or sevoflurane-fentanyl anesthesia. Anesthesiology. 2003;98:329–336.
51. Mielck F, Stephen H, Buhre W, et al. Effects of 1 MAC desflurane on cerebral metabolism, blood flow and carbon dioxide reactivity in humans. Br J Anaesth. 1998;81:155–160.
52. Fraga M, Rama-Maceiras P, Rodino S, et al. The effects of isoflurane and desflurane on intracranial pressure, cerebral perfusion pressure, and cerebral arteriovenous oxygen content difference in normocapnic patients with supratentorial brain tumors. Anesthesiology. 2003;98:1085–1090.
53. Lotto ML, Banoub M, Schubert A. Effects of anesthetic agents and physiologic changes on intraoperative motor evoked potentials. J Neurosurg Anesthesiol. 2004;16:32–42.
54. Akeson J, Didricksson I. Convulsions on anaesthetic induction with sevoflurane in young children. Acta Anaesthesiol Scand. 2004;48:405–407.
55 Sumitani M, Uchida K, Yasunaga H, et al. Prevalence of malignant hyperthermia and relationship with anesthetics in Japan: data from the diagnosis procedure combination database. Anesthesiology. 2011;114:84–90.
56 Brady JE, Sun LS, Rosenberg H, et al. Prevalence of malignant hyperthermia due to anesthesia in New York state, 2001-2005. Anesth Analg. 2009;109:1162–1166.
57 Rosenberg H, Shutack JG. Variants of malignant hyperthermia. Special problems for the paediatric anesthesiologist. Paediatr Anaesth. 1996;6:87–93.
58 Rosenberg H, Davis M, James D, et al. Malignant hyperthermia. Orphanet J Rare Dis. 2007;2:21.
59. Martin JL. Volatile anesthetics and liver injury: a clinical update or what every anesthesiologist should know. Can J Anesth. 2005;52:125–129.
60. Njoku D, Laster MJ, Gong DH, et al. Biotransformation of halothane, enflurane, isoflurane and desflurane to trifluoroacetylated liver proteins: association between protein acetylation and hepatic injury. Anesth Analg. 1997;84:173–178.
61. Frink EJ, Ghantous H, Malan TP, et al. Plasma inorganic fluoride with sevoflurane anesthesia: correlation with indices of hepatic and renal function. Anesth Analg. 1992;74:231–235.
62. Kharasch ED. Adverse drug reactions with halogenated anesthetics. Clin Pharmacol Ther. 2008;84:158–162.
Author: Rachel Eshima McKay